Phet Build an Atom Worksheet Answers Guide

Introduction to PhET's Build an Atom Simulation

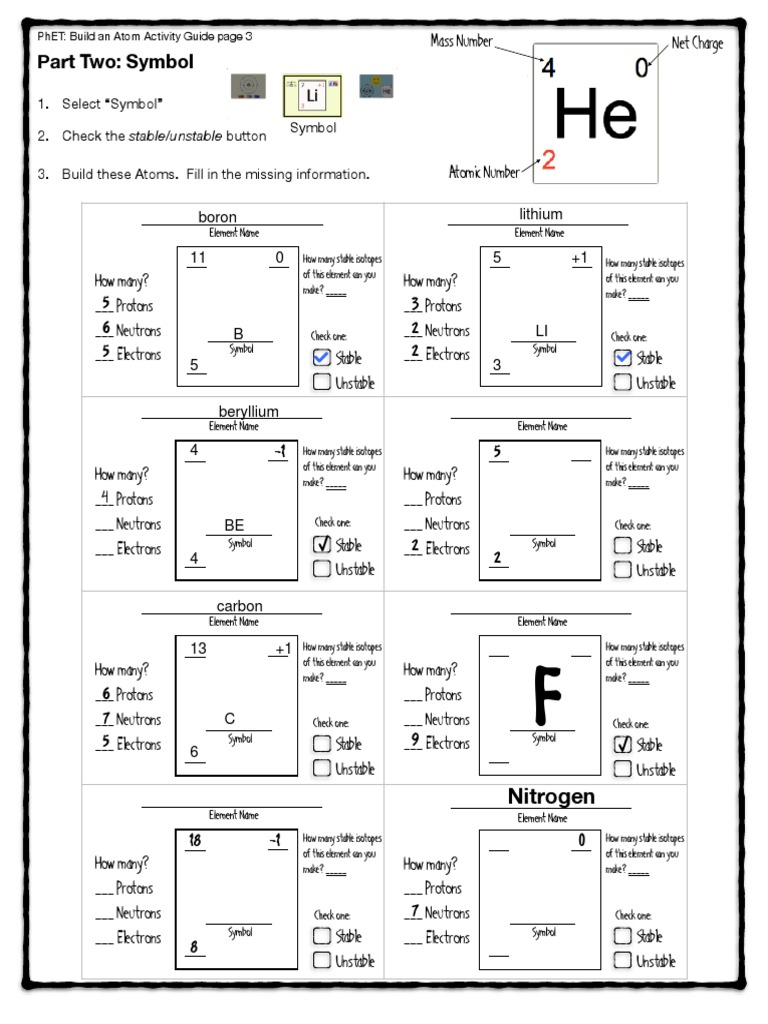
The PhET simulation “Build an Atom” provides an interactive and educational environment where students can learn about the structure of atoms, isotopes, and ions. Developed by the University of Colorado Boulder, this tool helps visualize complex atomic structures and understand fundamental chemistry concepts. In this comprehensive guide, we will explore how to use the simulation effectively, what each component means, and how to interpret the results from the PhET Build an Atom worksheet.
Understanding Atomic Structure

Before diving into the simulation, it’s crucial to grasp some basic concepts:
- Protons: Positively charged particles found in the nucleus of an atom. Their number determines the element.
- Neutrons: Neutral particles in the nucleus that contribute to the mass of an atom.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels.
How to Start with PhET Build an Atom

Here’s a step-by-step guide to get started:
- Open the PhET website and locate the "Build an Atom" simulation.
- Launch the simulation by clicking on the start button or link provided.
- Familiarize yourself with the interface:
- The atom model on the left where you can add or remove protons, neutrons, and electrons.
- The periodic table on the right for reference.
- Various tabs for different scenarios like "Build An Atom" or "Symbol".
🔬 Note: Ensure you have a stable internet connection as the simulation runs online.
Exploring Atomic Configuration

- Add or Remove Protons, Neutrons, Electrons:
- Use the buttons or drag-and-drop to add or subtract particles.
- Change the Element:
- Observe how adding or removing protons changes the element.
- Observe the Symbol:
- Note the atomic symbol, atomic number, and mass number changes with added or removed particles.
Worksheet Answers and Analysis

The PhET worksheet typically includes questions to test understanding. Here are some sample answers:
Q1. What changes when you add a proton to an atom?

Adding a proton:
- Changes the atomic number, hence the element itself.
- Affects the overall charge if the number of electrons doesn’t change accordingly.
Q2. How can you create an isotope?

- Add or remove neutrons to change the mass number while keeping the number of protons constant.
Q3. Explain how ions are formed.

Ions are formed by:
- Adding or removing electrons, thus altering the atom’s charge.
Q4. What happens if you remove all the neutrons?

- The atom becomes highly unstable due to lack of nuclear binding force provided by neutrons.
⚛️ Note: In real atoms, this situation would lead to rapid decay or fusion with other particles.
Practical Applications
- Nuclear Reactions: Simulate alpha and beta decay by manipulating neutrons and protons.
- Chemical Bonding: Understand how ions form compounds by altering electron counts.
- Periodic Trends: Explore trends in ionization energy, atomic radius, etc., by observing changes in electron shells.
Enhancing Learning Experience

Here are ways to make learning with “Build an Atom” more engaging:
- Group Activities: Collaborate on building molecules or elements.
- Real-World Scenarios: Simulate real-life isotopes used in medicine or industry.
- Creative Challenges: Design hypothetical atoms with unique properties.
To wrap up, using the PhET Build an Atom simulation not only simplifies complex atomic concepts but also makes learning interactive and fun. By manipulating protons, neutrons, and electrons, students can visualize and understand the building blocks of matter. This hands-on approach reinforces theoretical knowledge, providing a deeper understanding of atomic structure, isotopes, and ions. Moreover, by linking these concepts to everyday applications, the simulation bridges the gap between theoretical chemistry and practical science.
How can the PhET simulation help with understanding periodic trends?

+
The simulation allows users to add or remove electrons, demonstrating how the electron configuration affects atomic properties like electronegativity, ionization energy, and atomic radius, thus illustrating periodic trends.
Can students see the effects of changing electron shells?

+
Yes, by adjusting the number of electrons, students can observe how atoms fill different energy levels, which provides insight into electron configuration rules and the shell structure of atoms.
Is it possible to simulate ionic bonding with this tool?

+
Indirectly, yes. By creating ions through adding or removing electrons and then using the “Build a Molecule” tab, one can simulate how ions come together to form ionic compounds.