5 Expert Tips for Phet Atom Builder Worksheet Answers

Welcome to this comprehensive guide on Phet Atom Builder Worksheet Answers. If you're a student grappling with the complexities of atomic structure or an educator looking for effective teaching resources, this article will provide valuable insights. Here, we'll delve into expert tips that help in understanding and solving worksheets using the PhET Atom Builder simulation, enhancing both learning and teaching experiences.
Understanding PhET Simulations

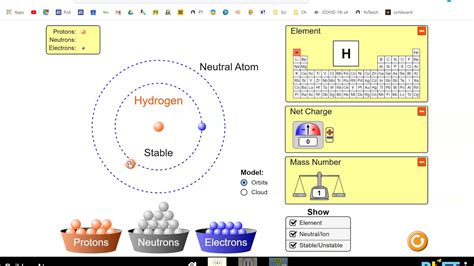
The PhET Interactive Simulations, developed by the University of Colorado Boulder, are designed to transform learning through interactive, research-based science and mathematics simulations. The Atom Builder simulation is particularly useful for visualizing how protons, neutrons, and electrons interact to form atoms.
🌟 Note: PhET simulations are free to use and accessible online, making them an excellent resource for both online and traditional classroom settings.
Expert Tips for Navigating the Atom Builder Worksheet

1. Master the Basics of the Simulation

Before diving into the worksheet questions, familiarize yourself with the Atom Builder simulation:
- Add and Remove Particles: Click on the plus and minus signs to add or remove protons, neutrons, and electrons. Observe how the changes affect the atom’s mass, charge, and identity.
- Use the Symbol and Name Key: This tool helps identify the element based on the number of protons, providing a direct correlation between atomic number and element identity.
- Understand Subatomic Particle Behavior: Pay attention to how electrons are arranged around the nucleus to form different orbitals.
2. Analyze Worksheet Questions Systematically

When solving worksheet problems, follow this systematic approach:
- Read Carefully: Understand what each question is asking. Identify if it’s about mass, charge, element identity, or electron configuration.
- Set Up the Atom: Use the simulation to replicate the atom described in the question. Adjust the particles until you match the provided information.
- Check Your Work: Use the element key to verify your setup. Ensure the simulation matches the expected results from your calculations.
✅ Note: Always verify both the numbers and the configuration to avoid common mistakes like mis-counting particles.
3. Use Interactive Features to Visualize Electron Shells

Understanding electron configuration is crucial:
- Electron Shells: Watch how electrons fill in shells, following the Aufbau principle. The visual representation aids in conceptual understanding.
- Orbital Diagrams: For advanced learners, simulations can be used to show electron configurations in terms of s, p, d, and f orbitals.
4. Experiment with Isotopes and Ions

Many worksheets will challenge students with isotopes or ions:
- Isotopes: Add or remove neutrons to change the mass of the atom without altering its chemical properties.
- Ions: Adjust the number of electrons to create positive or negative ions. Note how this impacts the atom’s charge and overall stability.
5. Integrate Real-World Examples

Relate the atomic structure concepts to real-world applications:
- Chemical Reactions: Discuss how electron configurations can predict reactivity and bonding behavior.
- Nuclear Energy: Explore how changes in neutron numbers relate to nuclear reactions like fission and fusion.
By integrating these examples, students can see the practical implications of what they learn.
💡 Note: This approach not only helps in memorizing facts but also in understanding the ‘why’ behind atomic structures.
Common Misconceptions and How to Overcome Them

Students often have misconceptions about atomic structure. Here’s how to tackle them:
- Neutrons Do Not Define Elements: Students should understand that it’s the number of protons that identifies an element, not the neutrons.
- Electron Configuration: Electron shells fill in a particular order, and the simulation can help correct the common mistake of placing electrons randomly.
Summary

The journey through understanding atomic structures with PhET Atom Builder simulation can be both educational and fun. By mastering the basics, analyzing questions systematically, visualizing electron configurations, experimenting with isotopes and ions, and integrating real-world examples, learners can deepen their understanding of atomic theory. This comprehensive approach not only aids in better scores on worksheets but also in a greater appreciation of chemistry and physics. By addressing common misconceptions and providing visual, interactive learning tools, we ensure that students not only memorize but truly understand the underlying principles of atomic structure.
Can PhET Simulations be used at home?
+
Yes, PhET simulations are freely accessible online, making them an excellent resource for home-based learning.
What if the simulation does not match my worksheet’s answer?

+
Ensure you’ve correctly interpreted the question. Check if all parameters like mass, charge, and electron configuration are correctly set up. Re-read the question, possibly from a different perspective.
How can I use the simulation to prepare for a test?

+
Use the simulation to reinforce concepts, test various scenarios, and understand electron configurations. Practice by creating different elements, isotopes, and ions.