5 Answers to Common Phase Change Phenomena Questions

Introduction to Phase Changes

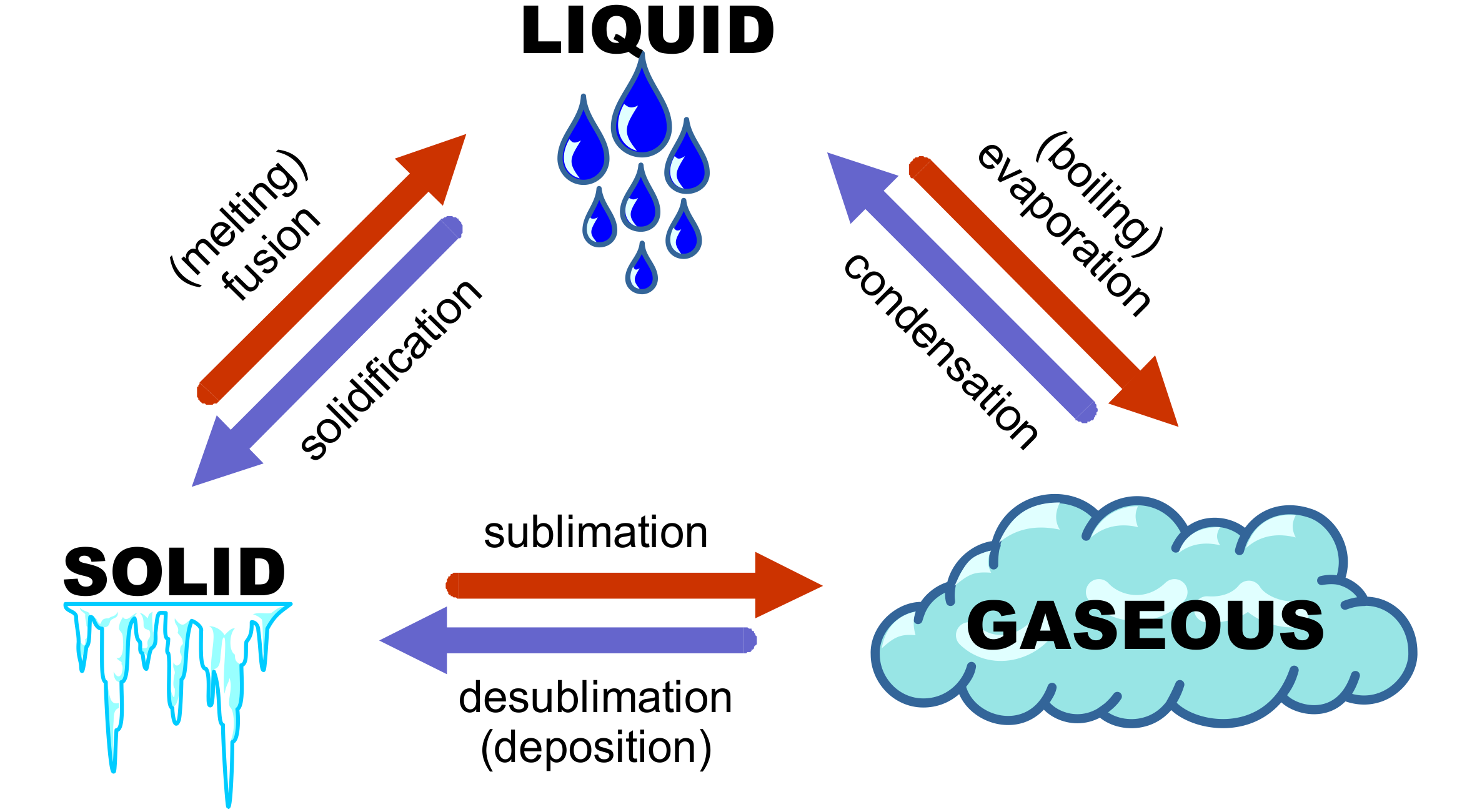
Phase changes in substances are fascinating events that occur daily around us, often without our explicit notice. Whether it’s the ice melting in your drink, the water boiling for your pasta, or the steam condensing back into water, these transformations are known as phase changes or transitions. This blog post will delve into five common phenomena related to phase changes, providing a clear understanding of these everyday but intricate processes.
1. Melting: Solid to Liquid

The first phase change we explore is melting. Melting occurs when a solid, like ice, is heated to its melting point and transforms into a liquid. Here’s how it works:
- Temperature: The melting point depends on the substance. For ice, this is 0°C (32°F). When ice is warmed to this temperature, it absorbs heat energy without an immediate rise in temperature, which is used to break the intermolecular bonds holding the solid structure.
- Heat Absorption: The absorbed heat disrupts the crystalline structure of the solid, converting it into a less ordered liquid state.
- Endothermic Process: Melting is an endothermic process, meaning it requires energy. This energy comes from the environment, often in the form of heat.
⚠️ Note: The melting point can change with pressure, which is why ice can melt at lower temperatures on mountain peaks due to lower atmospheric pressure.
2. Boiling: Liquid to Gas
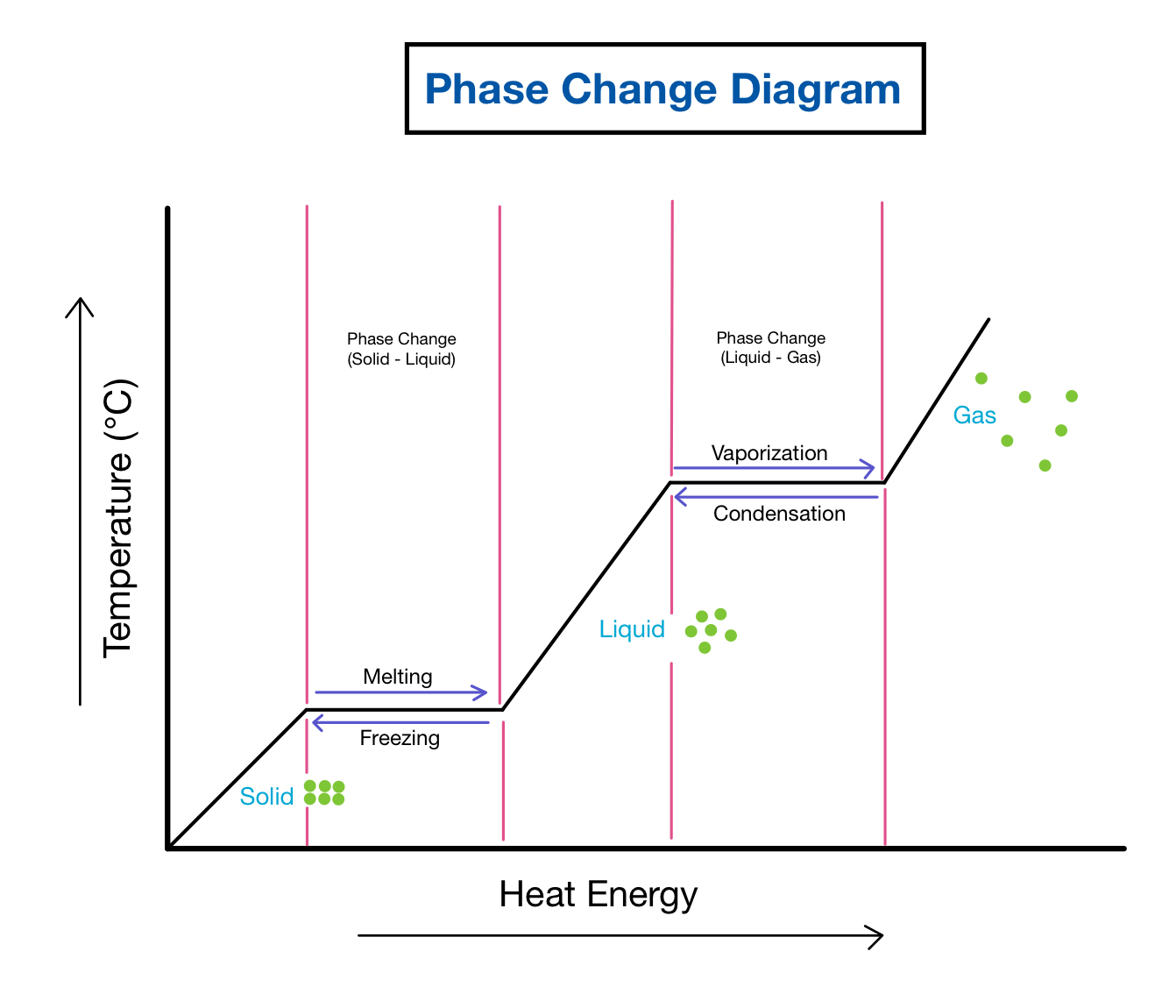
Next, we shift our focus to boiling, where a liquid changes into a gas. This process occurs at the boiling point of the liquid:
- Vapor Pressure: At the boiling point, the vapor pressure of the liquid equals the atmospheric pressure, allowing gas bubbles to form within the liquid.
- Convection Currents: As heat is applied, convection currents ensure that all parts of the liquid reach the boiling point, and bubbles of vapor rise to the surface, replacing liquid that turns into gas.
- Energy Consumption: Like melting, boiling is also endothermic. The heat energy increases molecular motion until the molecules escape into the gas phase.
3. Condensation: Gas to Liquid

When a gas cools, it can turn back into a liquid through the process known as condensation. This phenomenon is vital for our water cycle:
- Decrease in Temperature: As the gas cools, the molecules lose kinetic energy, slowing down and coming closer together.
- Loss of Intermolecular Space: When molecules get close enough, they can form intermolecular bonds, reverting the gas back to liquid form.
- Energy Release: Condensation is an exothermic process where the latent heat of vaporization is released back into the environment, often leading to phenomena like dew or fog.
4. Sublimation: Solid to Gas
Sublimation is the phase change where a solid directly turns into gas without passing through the liquid phase, a process often observed with substances like dry ice or mothballs:
- Direct Phase Change: Sublimation occurs when the temperature and pressure conditions allow a solid to convert directly to a gas, bypassing the liquid state.
- Energy Requirement: Though sublimation is endothermic, requiring energy, it’s less intuitive since there’s no liquid phase to absorb heat visibly.
- Applications: This process is crucial in preserving freeze-dried foods or in air fresheners where substances sublimation removes moisture.
🧠 Note: Sublimation can occur when the equilibrium vapor pressure of the solid exceeds the ambient pressure, often under reduced pressure.
5. Deposition: Gas to Solid

Opposite to sublimation, deposition involves the direct conversion of gas to solid. This phenomenon can be seen when frost forms on windows:
- Formation of Crystals: Gas molecules, upon losing enough energy, directly form crystals on surfaces, depositing as a solid without ever becoming a liquid.
- Weather Phenomena: Snowflakes are a well-known example where water vapor in the atmosphere turns directly into ice crystals.
- Energy Release: As with condensation, deposition releases latent heat, making the environment cooler in the process.
In understanding these phase changes, we not only learn about fundamental principles of thermodynamics but also appreciate how these processes impact our environment and daily life. Each phase change has its unique conditions and energy requirements, illustrating the fascinating behavior of matter under different conditions.
What causes ice to melt?

+
Ice melts when it is heated to its melting point (0°C or 32°F), where the heat energy breaks the hydrogen bonds holding the water molecules in a crystalline structure, turning them into a liquid.
Why does a pot of water boil faster at high altitudes?

+
At higher altitudes, the atmospheric pressure is lower, meaning the boiling point of water decreases. This lower boiling point reduces the energy needed to boil the water, so it happens faster.
How can condensation form on a glass of cold water?
+
When the temperature of the glass is lower than the dew point of the surrounding air, water vapor in the air condenses into liquid water upon contact with the cold surface, forming droplets on the outside of the glass.
What is the purpose of using dry ice in transport?

+
Dry ice is used to keep things cold during transport because, as it sublimates, it absorbs a significant amount of heat, cooling the environment around it.
How does deposition contribute to frost formation?

+
Frost forms through deposition when the temperature of a surface drops below the freezing point, allowing water vapor in the air to directly turn into ice crystals on contact.



