Master the Phase Change Diagram Worksheet Easily
Understanding Phase Change Diagrams

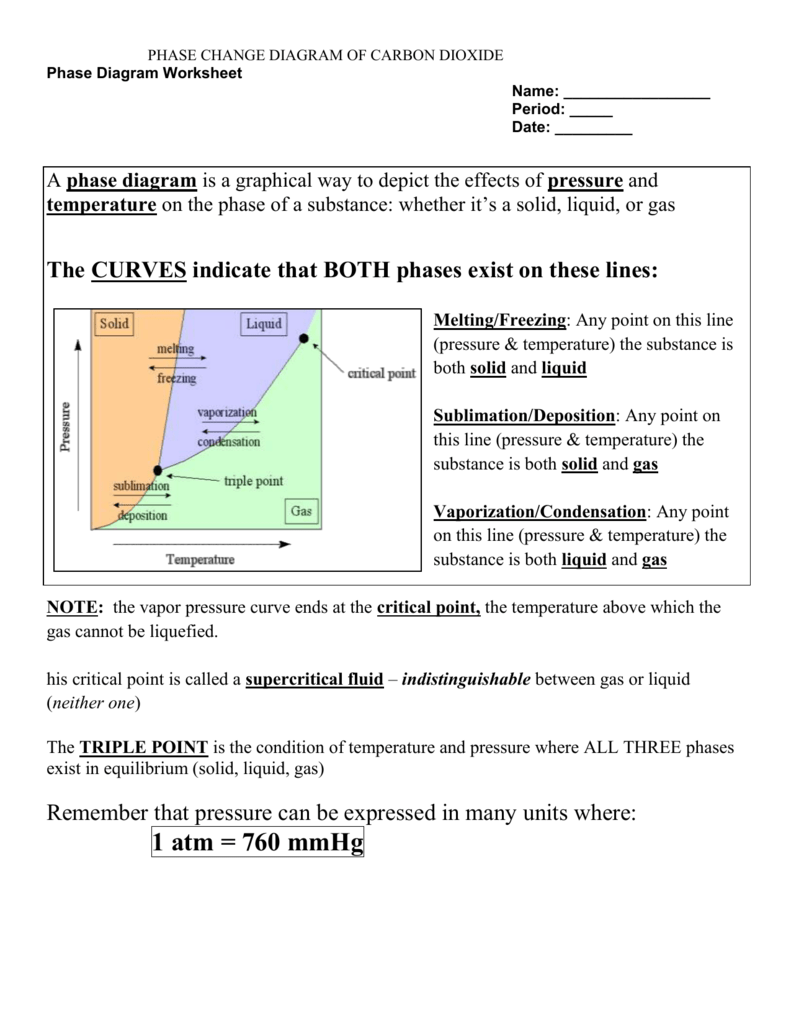
A phase change diagram, often referred to as a phase diagram, is a crucial tool in the study of physical chemistry and materials science. This diagram provides a graphical depiction of the conditions at which thermodynamically distinct phases (like solid, liquid, and gas) can occur, coexist, or transition between one another. It’s essential for understanding how substances behave under different conditions of pressure and temperature.
The Basics of Phase Diagrams

At the core of a phase change diagram are:
- Temperature: Plotted along the x-axis.
- Pressure: Plotted along the y-axis.
- Phase Boundaries: Lines that separate different phases on the diagram.
Each line on the phase diagram represents a point where two phases are in equilibrium. Here’s what they mean:
- Fusion Curve: The line where the solid and liquid phases coexist (melting/freezing).
- Vaporization Curve: The line where liquid and vapor phases coexist (boiling/condensation).
- Sublimation Curve: The line where solid directly turns into gas without becoming liquid (sublimation/deposition).
💡 Note: Some phase diagrams might include additional curves like the triple point, where all three phases coexist.
Mastering the Phase Change Diagram Worksheet

To excel in understanding and interpreting phase change diagrams, one needs to:
- Identify Phase Boundaries: Determine at what conditions different phases exist or transition into one another.
- Understand Triple Point: This is the unique point where all three phases can exist in equilibrium. Knowing how to locate and interpret the triple point is critical.
- Analyze Phase Transformations: Recognize how changes in temperature and pressure affect the phase of a substance.
Step-by-Step Guide to Reading the Diagram
- Find the conditions (temperature and pressure) at any point on the diagram.
- Follow the phase boundaries to see where phase transitions occur.
- Locate critical points, the end of the vaporization curve, and the triple point for complete understanding.
Practical Applications

Understanding phase change diagrams has several practical applications:
- Industrial Manufacturing: For example, determining conditions for the production of high-quality materials or optimizing chemical processes.
- Climate Science: Predicting how ice caps might react to changes in atmospheric pressure and temperature.
- Pharmaceuticals: Ensuring drugs remain stable under various storage conditions.
💡 Note: These diagrams can also help in the purification of substances through methods like distillation.
Summary of Key Points

To master phase change diagrams, one must grasp the fundamentals of temperature and pressure effects, locate and interpret phase boundaries, and understand phase transformations. This knowledge isn’t just academic; it applies directly to various industrial and scientific fields where controlling phase transitions is crucial.
What do the lines on a phase diagram represent?

+
The lines on a phase diagram denote the conditions at which two phases are in equilibrium. These include the fusion curve (solid-liquid equilibrium), vaporization curve (liquid-vapor equilibrium), and sublimation curve (solid-vapor equilibrium).
How can phase diagrams be used in industry?

+
Industries use phase diagrams to determine optimal conditions for material synthesis, chemical reactions, and storage conditions for various substances to ensure stability and desired properties.
What is the triple point, and why is it significant?

+
The triple point is a unique set of conditions (temperature and pressure) at which all three phases (solid, liquid, and gas) of a substance coexist in equilibrium. It’s significant because it can indicate critical points for phase transition behaviors and is used as a reference in scientific research.