5 Essential Tips for Mastering pH Calculations

Understanding and mastering pH calculations is essential for anyone involved in chemistry, biology, environmental science, or any other field where pH levels are critical. pH, which measures the acidity or alkalinity of a solution, is pivotal in ensuring chemical reactions proceed as expected, maintaining biological systems, and preserving environmental quality. Here are five comprehensive tips designed to help you become proficient in pH calculations:
1. Grasp the Concept of pH

At its core, pH stands for 'potential of hydrogen' and indicates the concentration of hydrogen ions (H+) in a solution:
- Acidic Solutions: Have a pH less than 7.0. The lower the pH, the higher the H+ concentration.
- Neutral Solutions: pH = 7.0, where the concentration of H+ and OH- (hydroxide ions) is equal.
- Basic (Alkaline) Solutions: Have a pH greater than 7.0, with decreasing H+ concentration as pH rises.
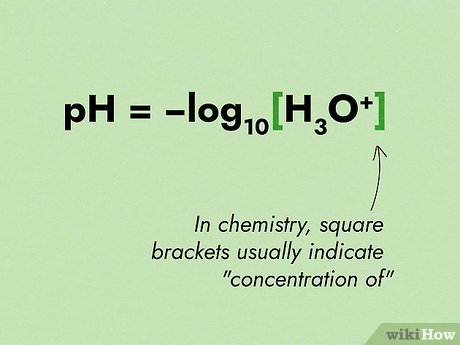
The pH can be calculated using the formula:
pH = -log[H+]
🔍 Note: Here, [H+] represents the molar concentration of hydrogen ions. A logarithm base 10 is used in the calculation.
2. Use a pH Meter Correctly

A pH meter offers the most accurate way to measure pH:
- Calibrate your pH meter using buffer solutions (usually at pH 4.01, 7.00, and 10.00) before taking any readings.
- Ensure the electrode is cleaned and properly soaked in the storage solution.
- Stir solutions gently to avoid air bubbles, which could impact readings.
- Perform the measurement at room temperature, or account for temperature variations.
- Keep the electrode vertically in the solution to allow for an accurate, stable reading.
⚠️ Note: Improper use of a pH meter can result in misleading or inaccurate pH values.
3. Practice With Common Examples

Start with exercises involving straightforward pH calculations:
- Hydrochloric Acid (HCl): Determine the pH of 0.1 M HCl:
pH = -log(0.1) = 1
- Sodium Hydroxide (NaOH): Calculate the pOH for 0.001 M NaOH and derive the pH:
pOH = -log(0.001) = 3 pH = 14 - pOH = 14 - 3 = 11
- Buffers: Practice calculating the pH of buffer solutions using the Henderson-Hasselbalch equation:
pH = pKa + log[Base] / [Acid]
4. Consider the Effects of Temperature

Temperature impacts pH measurements:
- Most pH meters are designed for 25°C. Use a temperature compensation feature or apply a correction factor if measuring at different temperatures.
- Remember that water's autoprotolysis constant (Kw) changes with temperature, affecting the ion product of water and the pH of neutral solutions.
🔥 Note: Higher temperatures generally lower the pH of pure water, as the dissociation of water increases.
5. Engage with pH Indicators
Visual indicators offer another way to estimate pH:
- Use universal pH indicators that change color over a wide pH range for quick approximations.
- Be aware that these indicators can be less accurate than a pH meter but are still useful for educational purposes or rapid field checks.
- Some indicators, like bromothymol blue, are specific to certain pH ranges and are useful when you expect pH within that range.
🧪 Note: Indicators can react differently based on solution strength or presence of interfering substances.
In essence, mastering pH calculations requires not only understanding the theoretical principles but also the practical application of these theories through tools like pH meters and indicators. Through consistent practice and application of these tips, you'll enhance your ability to manipulate and interpret pH, ensuring accuracy and reliability in your measurements.
Why is it necessary to calibrate a pH meter?

+
Calibration ensures the pH meter accurately reflects the true pH of the solution, compensating for any sensor drift or temperature changes.
What are the common mistakes in pH measurement?

+
Errors often arise from poor calibration, temperature variances not accounted for, not stirring the solution, and using old or contaminated electrode solutions.
How does the presence of salts affect pH readings?

+
Salts like sodium chloride can influence the ionic strength of the solution, which might lead to slight alterations in the electrode’s performance and pH reading accuracy.
What are the implications of not accounting for temperature in pH measurements?

+
Ignoring temperature changes can lead to significant errors in pH measurements since pH is temperature-sensitive. Adjustments or temperature compensation are crucial.
Can pH indicators be used for precise pH determination?

+
While pH indicators can give a rough estimate, they are not as precise as pH meters. They are best used for quick checks or educational purposes where exact precision is not critical.