Master pH and pOH with Our Easy Worksheet Answers

pH and pOH are fundamental concepts in chemistry, often studied in high school and college chemistry courses. Understanding these concepts is crucial for a wide array of fields, from environmental science to pharmaceutical research. This detailed guide provides an in-depth look at pH and pOH, how to master their calculations, and how to effectively use worksheets to reinforce your learning. Let's dive into the world of acid-base balance!
Understanding pH and pOH

pH stands for the “power of hydrogen” and it measures the acidity or alkalinity of a solution. The pH scale ranges from 0 to 14:
- pH of 0 to 6: Acidic
- pH of 7: Neutral
- pH of 8 to 14: Basic or Alkaline
Conversely, pOH measures the alkalinity of a solution by assessing the concentration of hydroxide ions (OH-). The relationship between pH and pOH is straightforward:
💡 Note: pH + pOH = 14 for any aqueous solution at 25°C.
How to Calculate pH and pOH
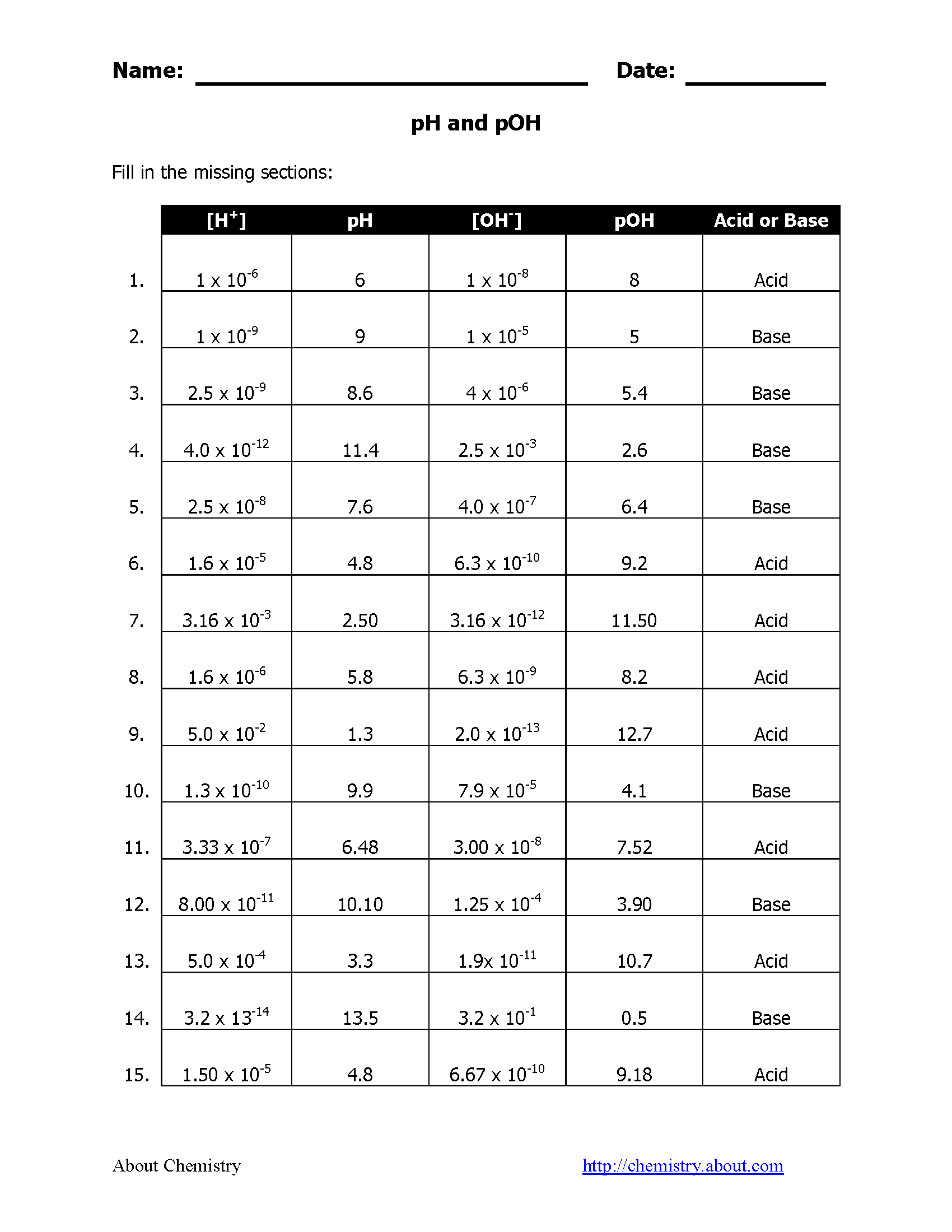
The calculation of pH and pOH is relatively simple when you understand the underlying principles:
1. From Hydrogen Ion Concentration (H+)

- pH = -log[H+]
- To calculate pOH, you first find the hydroxide ion concentration from the Kw (water dissociation constant, 1.0 x 10-14 at 25°C) and then apply the log formula.
2. From Hydroxide Ion Concentration (OH-)

- pOH = -log[OH-]
- Then pH is determined by the relationship:
- pH = 14 - pOH
| Acid or Base | [H+] | pH | [OH-] | pOH |
|---|---|---|---|---|
| HCl (strong acid) | 0.1 M | 1 | 1x10^-13 M | 13 |
| NaOH (strong base) | 1x10^-14 M | 14 | 0.1 M | 0 |

Mastering pH and pOH with Worksheets

Worksheets are excellent tools for mastering pH and pOH calculations:
Identify Key Information

When faced with a worksheet question:
- Identify whether the solution is acidic or basic.
- Look for given concentrations of H+, OH-, or pH/pOH values.
Solving pH/pOH Problems

Here’s how to approach different types of questions:
- Given [H+]: Use the formula pH = -log[H+].
- Given [OH-]: Calculate pOH first, then use pH + pOH = 14 to find pH.
- Given pH or pOH: Convert to [H+] or [OH-] using the inverse log.
🔍 Note: Practice worksheets with a variety of scenarios to ensure you're comfortable with all aspects of pH and pOH calculations.
Real-World Applications

Understanding pH and pOH isn’t just an academic exercise; they have practical applications:
Environmental Science

- Monitoring water quality in rivers, lakes, and oceans.
- Assessing soil pH to determine suitable crops or the need for amendments.
Health and Medicine

- Determining the pH of bodily fluids to diagnose conditions like acidosis or alkalosis.
- Formulating medications with the correct pH to ensure they work effectively in the body.
Industry

- Quality control in food production, where pH affects taste, shelf life, and microbial growth.
- Process control in manufacturing, from metal treatment to paper production.
Effective Tips for Learning pH and pOH

To excel in pH and pOH:
- Regular Practice: Use worksheets to reinforce your understanding. Repetition helps build confidence.
- Understand the Concepts: Don't just memorize formulas. Grasp why pH and pOH are important and how they relate.
- Use Visual Aids: Draw pH scales or concentration diagrams to visualize the process.
- Link to Real-World Examples: Relate chemical calculations to practical situations for better retention.
By taking these steps, you'll develop a solid foundation in understanding and applying pH and pOH calculations to various scenarios.
In this comprehensive guide, we've delved deep into the core concepts of pH and pOH, explored their calculations, and discussed how worksheets can help in mastering these topics. We've seen their practical applications in various fields, emphasizing the importance of a thorough understanding. With regular practice and a focus on conceptual understanding, mastering pH and pOH becomes a manageable goal, paving the way for success in chemistry and beyond.
What is the significance of pH and pOH in everyday life?

+
pH and pOH are crucial in everyday life as they help in determining the acidity or alkalinity of substances. This knowledge is essential for cooking, health care (such as testing urine pH), environmental monitoring, and even in personal care products like shampoos and conditioners where the pH affects their effectiveness and gentleness on skin or hair.
How can one improve their ability to calculate pH and pOH?

+
Improving calculation skills for pH and pOH can be achieved through:
- Regular practice using worksheets and online quizzes.
- Understanding the logarithmic scale and how pH/pOH relate to ion concentrations.
- Memorizing key relationships like pH + pOH = 14.
- Using mnemonic devices or conceptual models to remember formulas.
Can pH and pOH change with temperature?

+
Yes, both pH and pOH can change with temperature because the ion product of water (Kw) varies with temperature. At 25°C, Kw is 1.0 x 10^-14, but this changes at different temperatures, affecting the pH and pOH calculations accordingly.