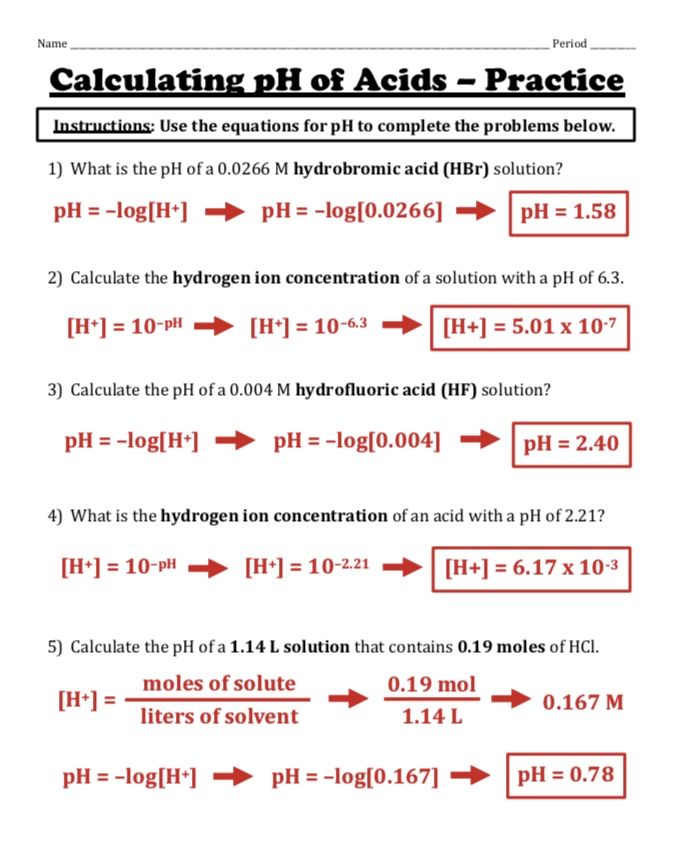
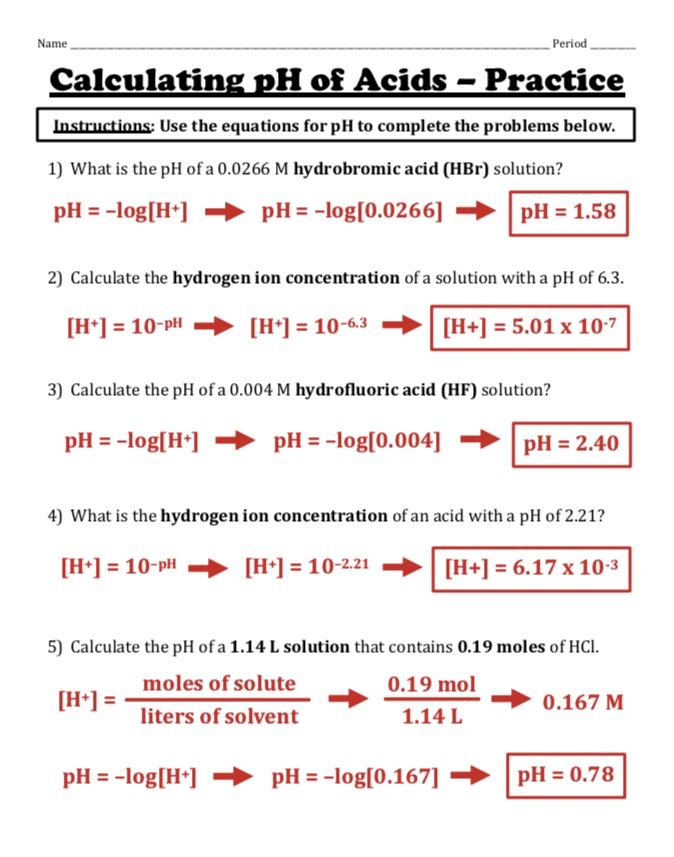
Mastering pH and pOH: 10 Essential Worksheet Answers
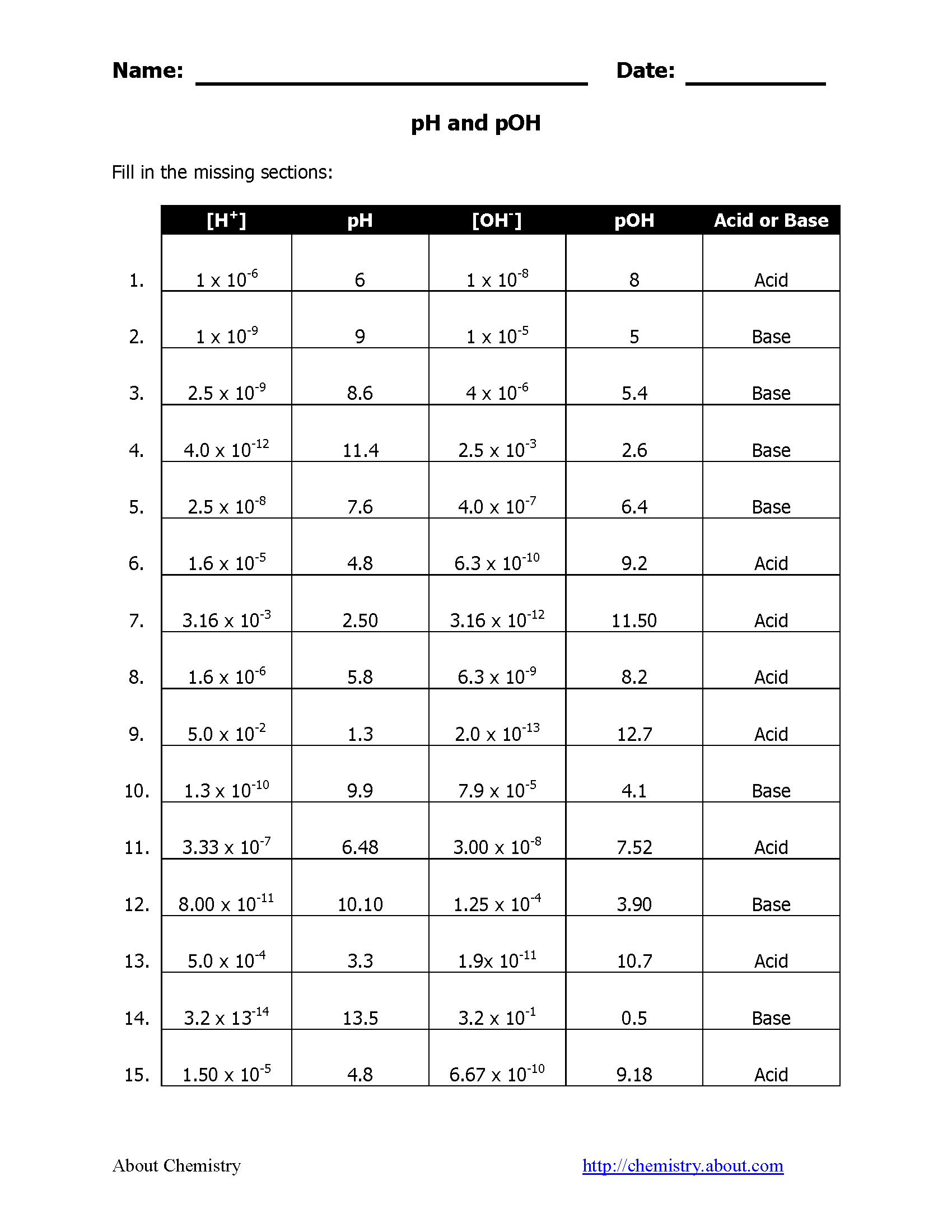
Understanding pH and pOH is crucial for students studying chemistry, particularly in the realm of acids and bases. These measurements help chemists determine the acidity or alkalinity of solutions, which has significant implications in various scientific fields. This comprehensive guide walks through ten essential worksheet answers to help students master these concepts effectively.
What is pH?
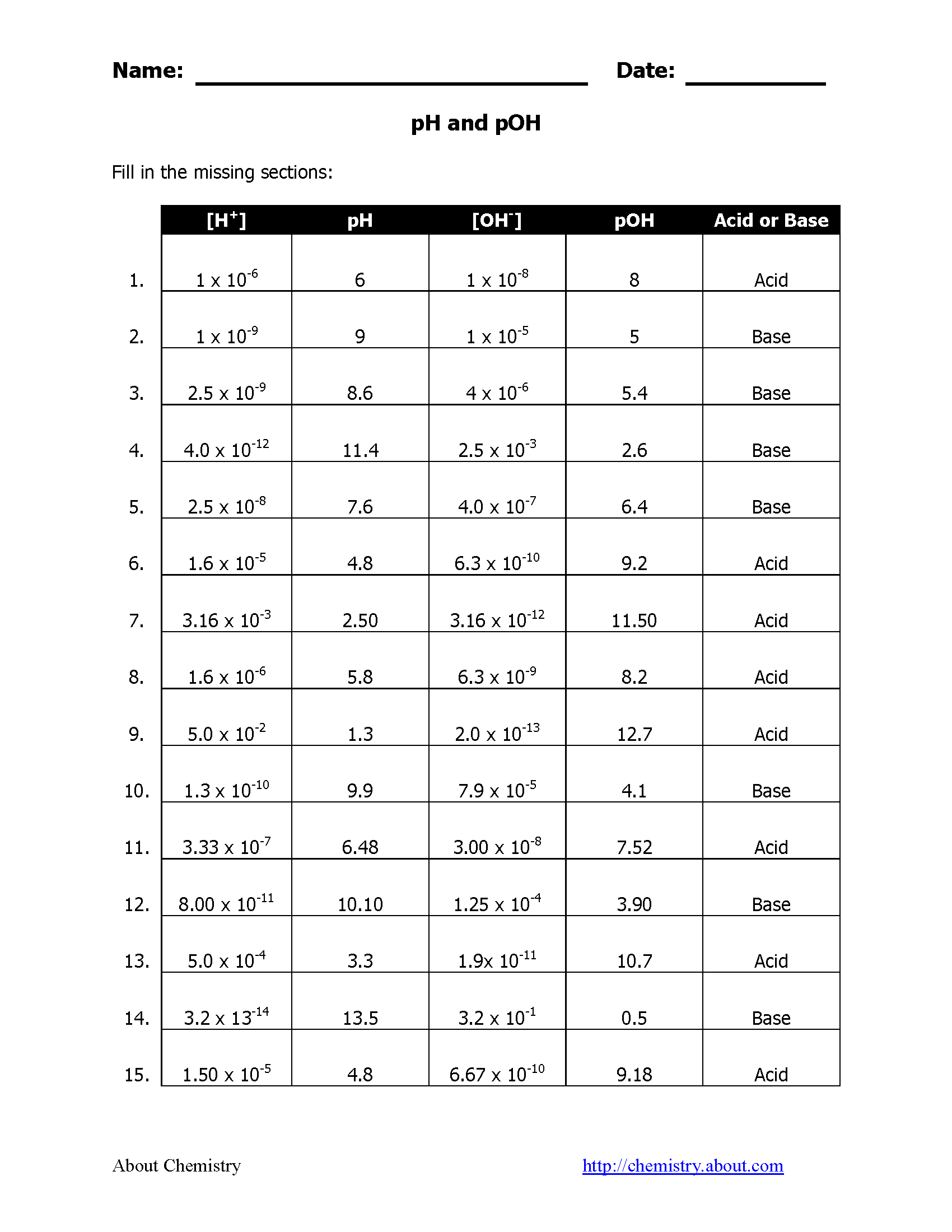
pH stands for "power of hydrogen," and it measures the activity of hydrogen ions (H+) in a solution. Here are key points about pH:
- The pH scale ranges from 0 to 14, where 7 is considered neutral.
- Values below 7 indicate acidity; the lower the number, the more acidic the solution.
- Values above 7 indicate alkalinity; the higher the number, the more alkaline the solution.
- pH is calculated using the formula: pH = -log[H+].
📝 Note: The logarithmic nature of the pH scale means that each pH unit represents a tenfold change in hydrogen ion concentration.
What is pOH?

Parallel to pH, pOH measures the activity of hydroxide ions (OH-) in a solution:
- The pOH scale also ranges from 0 to 14, where 7 is neutral for pure water at standard conditions.
- Values below 7 indicate more basic or alkaline solutions.
- Values above 7 indicate more acidic solutions.
- The formula for pOH is: pOH = -log[OH-].
How to Calculate pH from [H+]?
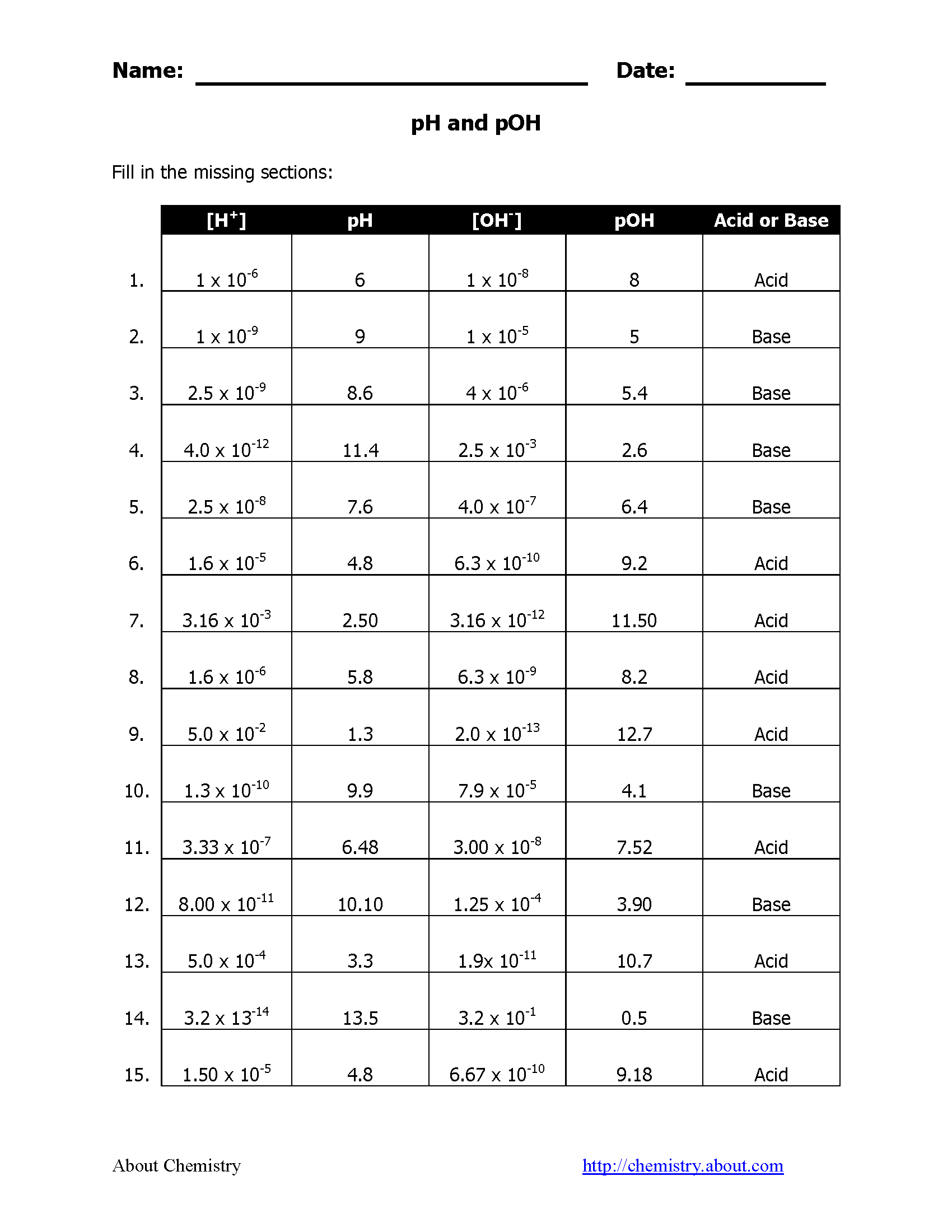
Calculating pH from the concentration of hydrogen ions is straightforward with this formula:
pH = -log[H+]
Example:
- If [H+] = 1 x 10-5 M, then:
- pH = -log(1 x 10-5) = 5
How to Calculate pOH from [OH-]?

Here's how you can calculate pOH from the concentration of hydroxide ions:
pOH = -log[OH-]
Example:
- If [OH-] = 1 x 10-7 M, then:
- pOH = -log(1 x 10-7) = 7
Relationship Between pH and pOH
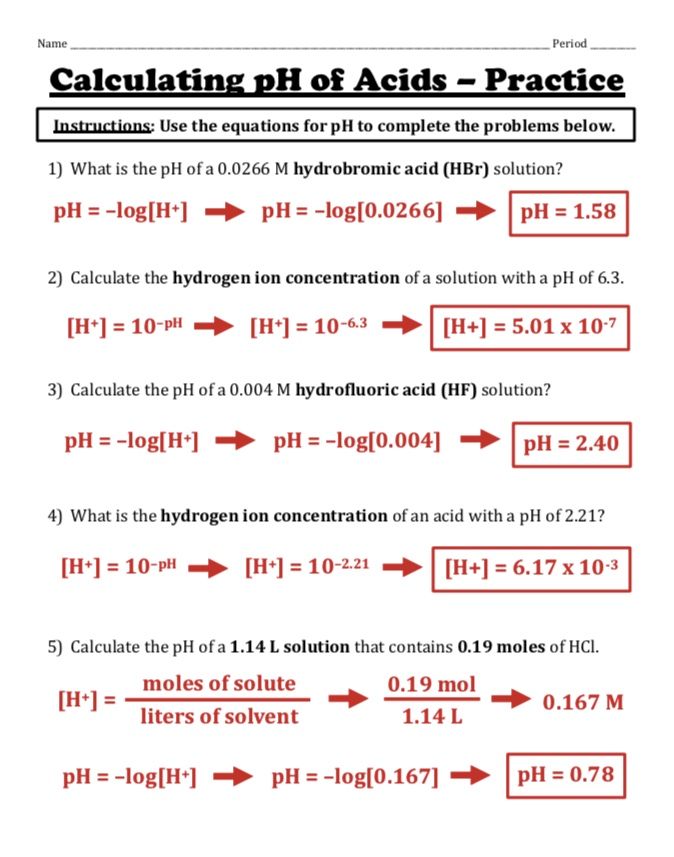
At 25°C in pure water, the relationship between pH and pOH is given by:
pH + pOH = 14
This relationship can be used to calculate one when the other is known:
- If pH is 12, then pOH is 14 - pH = 14 - 12 = 2
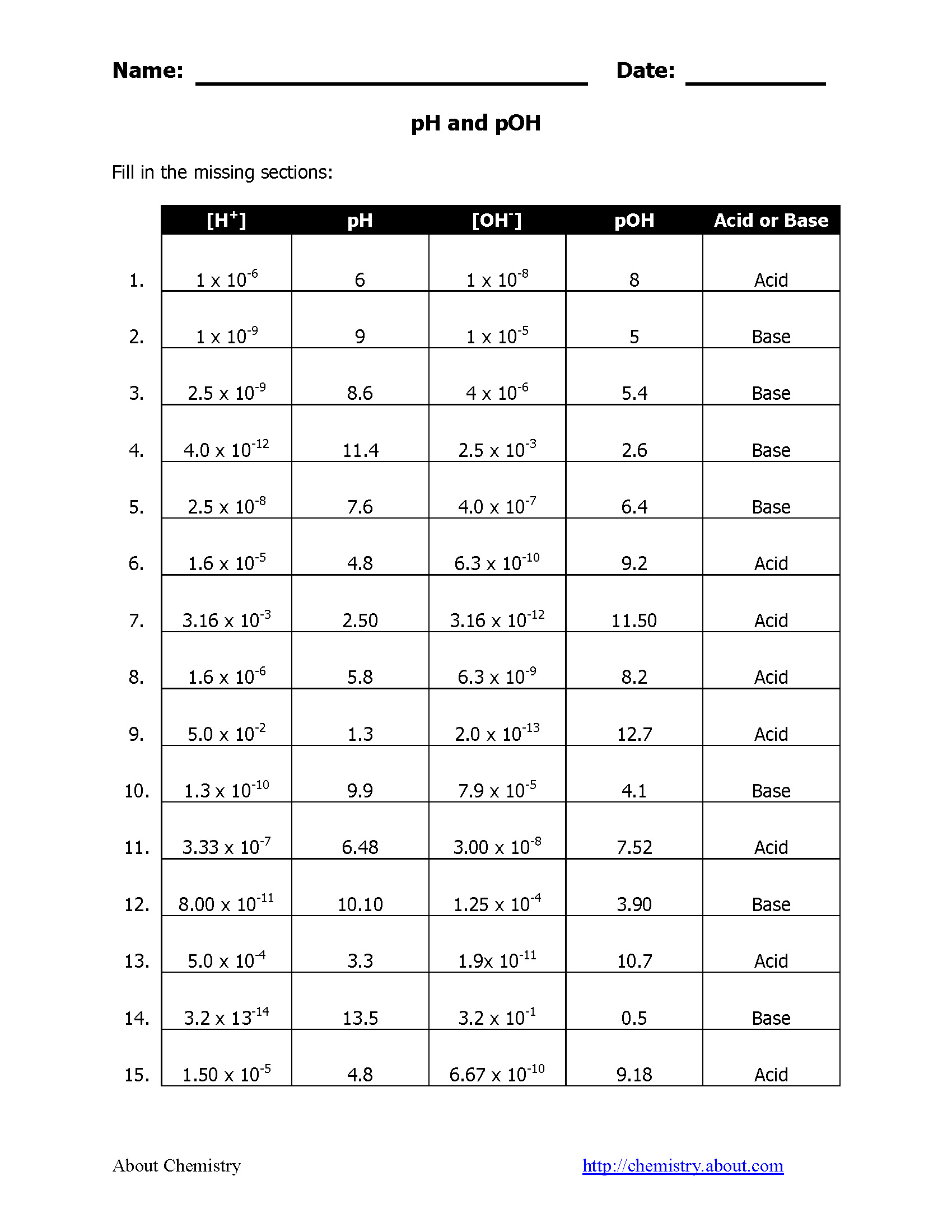
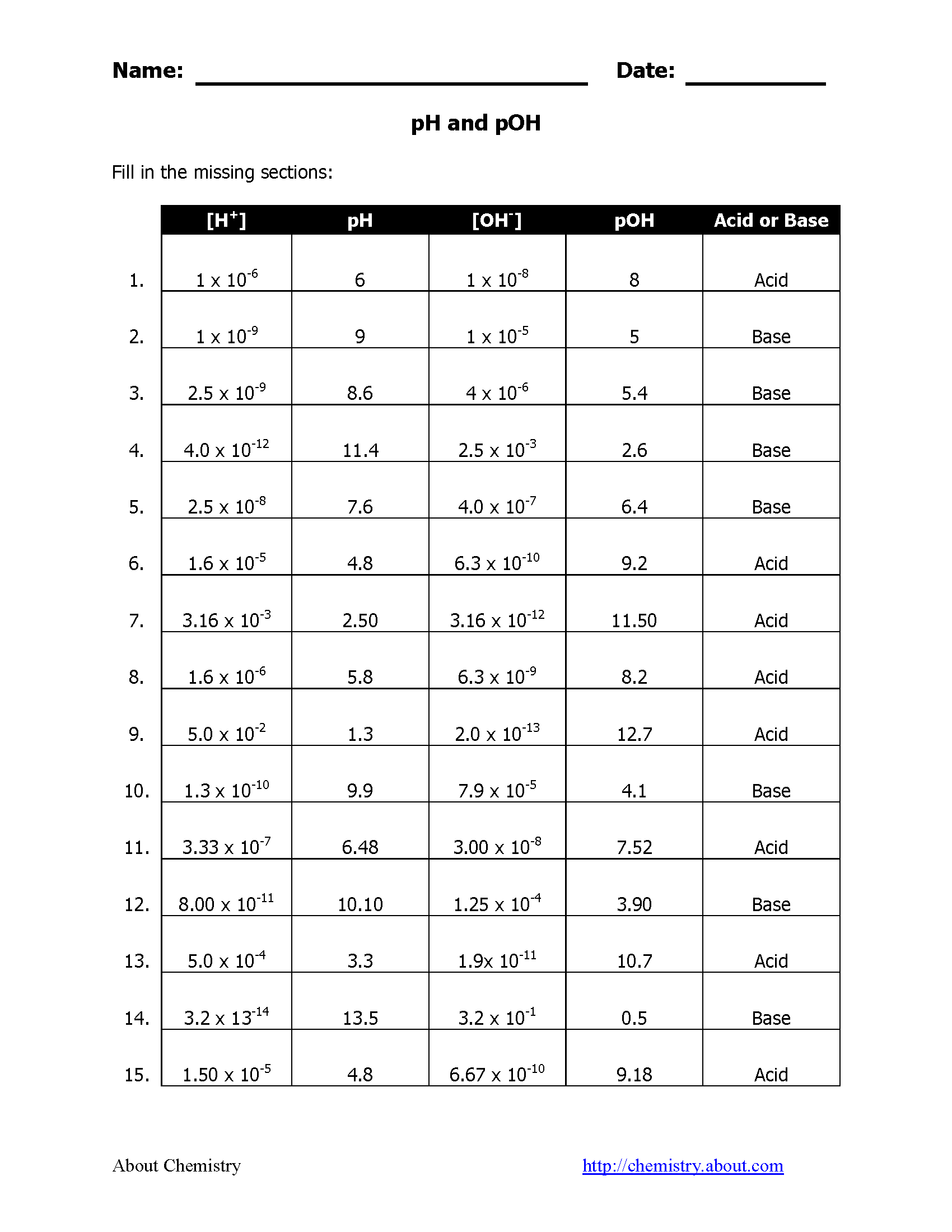
Understanding Acidic and Basic Solutions

Here's a table illustrating common pH values and their corresponding pOH values:
| Example | pH | pOH | Category |
|---|---|---|---|
| Battery Acid | 1 | 13 | Highly Acidic |
| Vinegar | 3 | 11 | Moderately Acidic |
| Pure Water | 7 | 7 | Neutral |
| Ammonia Solution | 11 | 3 | Moderately Basic |
| Sodium Hydroxide (caustic soda) | 14 | 0 | Highly Basic |

Key Questions and Answers

In this section, we'll address some common problems encountered in pH and pOH worksheets:
Q: If [H+] is 1.0 x 10-3 M, what is the pH?

A: Using the pH formula:
pH = -log(1.0 x 10-3) = 3
Q: What is the pOH if [OH-] is 4.0 x 10-10 M?
A: Apply the pOH formula:
pOH = -log(4.0 x 10-10) ≈ 9.398
Q: How can you find the pH of a solution with pOH = 8?

A: Use the relationship:
pH = 14 - pOH = 14 - 8 = 6
Q: If the pH of a solution is 6.5, what is the [H+]?
A: Since pH = -log[H+], rearrange the equation:
[H+] = 10^(-pH) = 10^(-6.5) ≈ 3.16 x 10-7 M
📝 Note: In real-world applications, pH might fluctuate due to temperature changes or other environmental factors.
Q: What is the concentration of OH- in a solution with pOH = 4?

A: pOH = -log[OH-] so:
[OH-] = 10^(-pOH) = 10^(-4) = 1.0 x 10-4 M
Summary
Throughout this guide, we've explored the essential concepts of pH and pOH, their calculations, and the relationship between them. Understanding these measurements provides a solid foundation in understanding acid-base chemistry, which is fundamental in various fields including environmental science, biology, and industrial processes. By mastering these calculations, students can confidently tackle complex chemical problems and gain insights into the behavior of chemical solutions.
What does a pH of 7 signify?
+
A pH of 7 indicates a neutral solution, where the concentration of hydrogen ions (H+) is equal to that of hydroxide ions (OH-).
How does temperature affect pH?

+
As temperature increases, the ionization of water increases, leading to a slight shift in pH, making it more acidic, but for pure water, pH remains 7 at all temperatures.
Why is pH important in daily life?

+
pH affects the taste, safety, and shelf-life of food; water quality; effectiveness of cleaning agents; biological processes, and more.
Can pH and pOH be used interchangeably?

+
While related, pH and pOH cannot be used interchangeably as they measure different ions; pH measures hydrogen ions, while pOH measures hydroxide ions.



