pH and pOH Practice Worksheet Answers Unveiled

Understanding pH and pOH is crucial for students of chemistry and anyone interested in the properties of acids and bases. These concepts are not only fundamental in understanding chemical reactions but also in practical applications like water treatment, pharmaceutical manufacturing, and environmental sciences. Here's a comprehensive guide to demystify pH and pOH, complete with practice worksheet answers and insightful explanations.
The Basics of pH and pOH

The pH scale measures the acidity or basicity of an aqueous solution. It ranges from 0 to 14, where:
- Acids have a pH less than 7.
- Bases have a pH greater than 7.
- pH 7 is neutral, typical of pure water.
The relationship between pH and pOH is given by the equation:
pH + pOH = 14
This equation holds at standard temperature and pressure, ensuring that in any aqueous solution, if you know one, you can calculate the other.
pH and pOH Calculations

To calculate pH from hydrogen ion concentration ([H+]):
- pH = -log [H+]
Similarly, pOH can be calculated from the hydroxide ion concentration ([OH-]):
- pOH = -log [OH-]
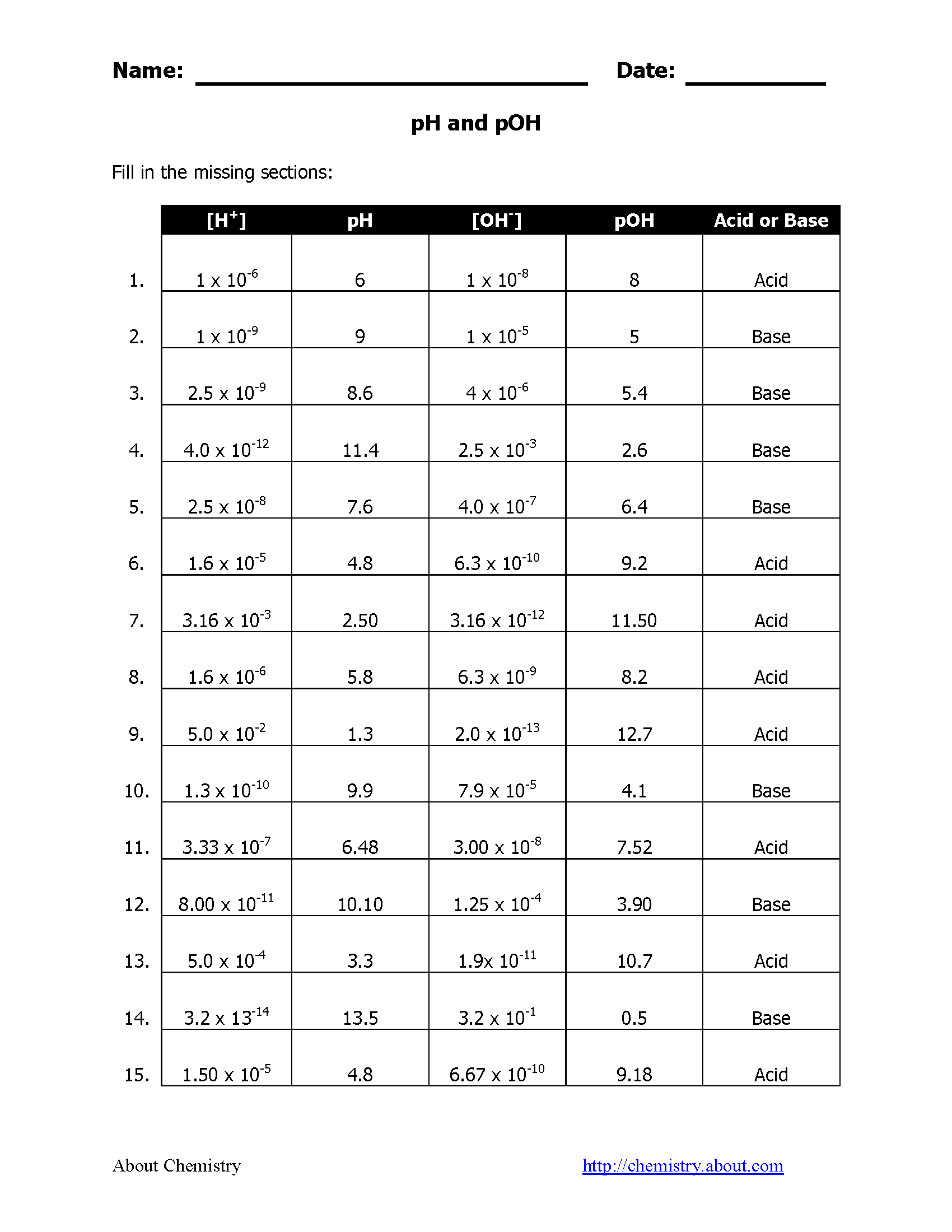
Practice Worksheet Answers

Let's delve into some common worksheet problems and provide the answers:
Problem 1:

Calculate the pH of a solution where the concentration of H+ ions is 1 x 10-4 M.
Answer: pH = -log [H+] = -log (1 x 10-4) = 4
Problem 2:

Find the pOH of a solution whose OH- ion concentration is 2.5 x 10-2 M.
Answer: pOH = -log [OH-] = -log (2.5 x 10-2) = 1.6
Problem 3:
Determine the pH of a solution when the pOH is 9.
Answer: pH + pOH = 14, so pH = 14 - pOH = 14 - 9 = 5
| Problem | Answer |
|---|---|
| pH when [H+] = 1 x 10-4 M | 4 |
| pOH when [OH-] = 2.5 x 10-2 M | 1.6 |
| pH when pOH = 9 | 5 |

💡 Note: Always check your logarithmic calculations for precision, especially when dealing with small or large numbers, to ensure your pH or pOH values are correct.
Understanding the Impact of pH and pOH

The pH and pOH of a solution have profound effects:
- They dictate the behavior of substances in solution, influencing solubility, reactivity, and enzymatic activity.
- In environmental science, pH is a key factor in water quality, soil health, and aquatic life.
- In the body, pH balance is crucial for maintaining proper physiological function; for example, blood pH is tightly regulated to remain close to 7.4.
Conclusion

In summary, pH and pOH are not just numbers on a scale; they encapsulate a wealth of information about the chemical properties of solutions. Understanding these concepts helps in predicting chemical behavior, ensuring safety in industrial applications, and even in daily life. By mastering the calculations and understanding the implications, one can better navigate the vast field of chemistry with confidence.
What is the importance of pH in daily life?

+
pH influences food taste, the effectiveness of cleaning agents, soil health for plant growth, and the safety of drinking water. Maintaining proper pH levels is essential for human health, industrial processes, and ecological balance.
Can pH affect the environment?

+
Absolutely, pH directly affects aquatic ecosystems by influencing the availability of nutrients and the toxicity of pollutants. Acid rain, caused by industrial pollution, can change the pH of soil and water, negatively impacting plant and animal life.
Why is pH important in medicine?

+
pH affects drug solubility and absorption in the body. It’s also crucial for controlling physiological conditions where slight changes can lead to serious health issues, like acidosis or alkalosis in blood pH.