Master PH and POH Calculations: Worksheet Answers Revealed

In the fascinating world of chemistry, understanding concepts like pH and pOH is fundamental for students and professionals alike. These terms are central to acid-base chemistry and are crucial for a variety of applications, from water purification to biological systems. This blog post aims to demystify pH and pOH calculations, providing a step-by-step guide with explanations and answers to common worksheet problems. Whether you're a student, a chemistry enthusiast, or a professional in need of a refresher, this post will equip you with the knowledge to tackle pH and pOH calculations confidently.
Understanding pH and pOH

Before diving into the calculations, let's clarify what pH and pOH represent:
- pH: It measures the acidity or alkalinity of a solution. The scale ranges from 0 to 14, where pH 7 is neutral, below 7 is acidic, and above 7 is basic.
- pOH: This represents the base strength, or how 'alkaline' a solution is. The formula for pOH is -log[OH-], which is parallel to pH in that pOH + pH = 14 in aqueous solutions at 25°C.

Basic pH and pOH Formulas

The basic formulas you need to know are:
- pH: pH = -log[H+]
- pOH: pOH = -log[OH-]
- Relationship: pH + pOH = 14
⚗️ Note: [H+] represents the concentration of hydrogen ions, and [OH-] represents the concentration of hydroxide ions in moles per liter.
Steps to Calculate pH

- Identify the concentration: Determine the concentration of the acid in moles per liter. This could be given or calculated from a titration experiment.
- Substitute in the formula: Use the pH formula, pH = -log[H+], where [H+] is the concentration.
- Perform the calculation: Take the negative logarithm to find the pH value.
Example: Calculating pH

Let's calculate the pH of a solution with a hydrogen ion concentration of 1.0 x 10-4 M:
pH = -log(1.0 x 10-4) pH = -(-4) = 4
Therefore, the pH of the solution is 4.
Steps to Calculate pOH

The steps for calculating pOH are very similar to those for pH:
- Identify the concentration: Find the concentration of OH- ions in moles per liter.
- Substitute in the formula: Use pOH = -log[OH-].
- Calculate: Apply the logarithm and take its negative value.
Example: Calculating pOH

Let's calculate the pOH of a solution with an OH- ion concentration of 2.5 x 10-10 M:
pOH = -log(2.5 x 10-10) pOH = -(-9.6) ≈ 9.6
The pOH of the solution is approximately 9.6.
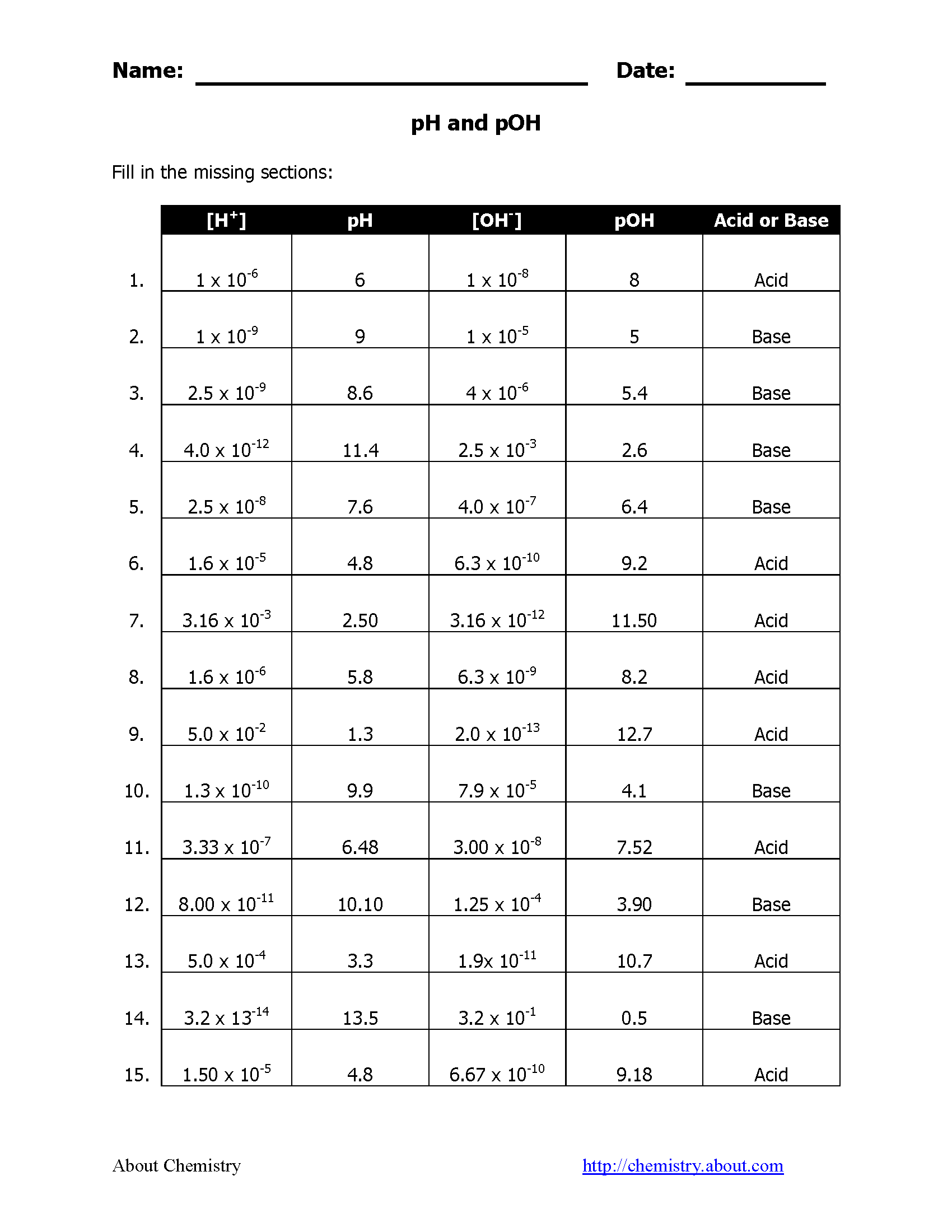
Worksheet Answers and Examples

Here are some common worksheet problems with their solutions to illustrate these concepts:
Problem 1: Calculate the pH of a solution with [H+] = 3.5 x 10-9 M

pH = -log(3.5 x 10-9) pH = 8.45
Problem 2: What is the pOH of a solution with [OH-] = 7.8 x 10-5 M?
pOH = -log(7.8 x 10-5) pOH = 4.11
With pOH calculated, we can find pH by using the relationship:
pH = 14 - pOH pH = 14 - 4.11 = 9.89
Problem 3: Determine the pH and pOH of water

Pure water dissociates to form equal amounts of H+ and OH- at 25°C, with [H+] = [OH-] = 1.0 x 10-7 M.
- pH = -log(1.0 x 10-7) = 7
- pOH = -log(1.0 x 10-7) = 7
Problem 4: If pH is 6.5, what are [H+] and [OH-]?

- [H+] = 10-6.5 = 3.16 x 10-7 M
- pOH = 14 - pH = 14 - 6.5 = 7.5
- [OH-] = 10-7.5 = 3.16 x 10-8 M
In summary, calculating pH and pOH involves understanding their definitions, applying the correct formulas, and converting between concentrations and logarithmic scales. This knowledge is essential for mastering acid-base chemistry, where pH and pOH not only define a solution's acidity or alkalinity but also dictate how substances interact within those conditions. This guide has provided you with the tools to solve basic pH and pOH problems, but remember that chemistry is an experimental science, and practical experience through lab work will reinforce these concepts.
What does a pH value of 7 signify?

+
A pH of 7 indicates a neutral solution, meaning the concentrations of hydrogen and hydroxide ions are equal at 25°C.
Can a pH or pOH value be negative?

+
Yes, negative pH or pOH values can occur when the concentration of H+ or OH- is greater than 1M. This usually occurs in highly acidic or alkaline conditions outside the standard scale.
How does temperature affect pH and pOH?

+
Temperature affects the dissociation of water, thus changing the pH and pOH values. Higher temperatures generally decrease pH as water dissociates more.