5 Essential Tips for pH and pOH Calculations

The measurement of pH and pOH are fundamental for understanding the acidity or basicity of aqueous solutions in chemistry. They provide a window into the chemical properties of substances, which is essential for fields ranging from environmental science to pharmaceuticals. Here, we delve into five indispensable tips to help you master pH and pOH calculations, ensuring you navigate these concepts with confidence and precision.
Understand the pH and pOH Scales

pH (potential of hydrogen) is a logarithmic scale used to specify the acidity or alkalinity of a solution, while pOH (potential of hydroxyl ion) indicates its basicity. Both scales range from 0 to 14:
- pH: A value less than 7 is acidic, 7 is neutral, and greater than 7 is alkaline (or basic).
- pOH: A value less than 7 is basic, 7 is neutral, and greater than 7 is acidic.
Knowing these scales provides a basic understanding of what the pH or pOH value means in real terms for a solution.
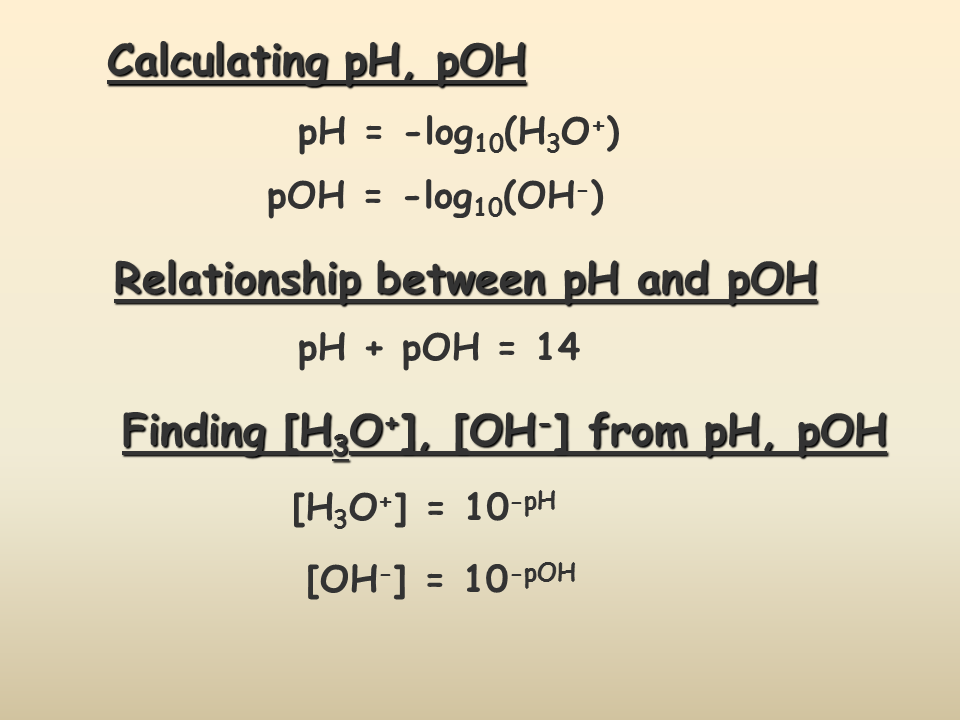
Know the pH and pOH Formulas
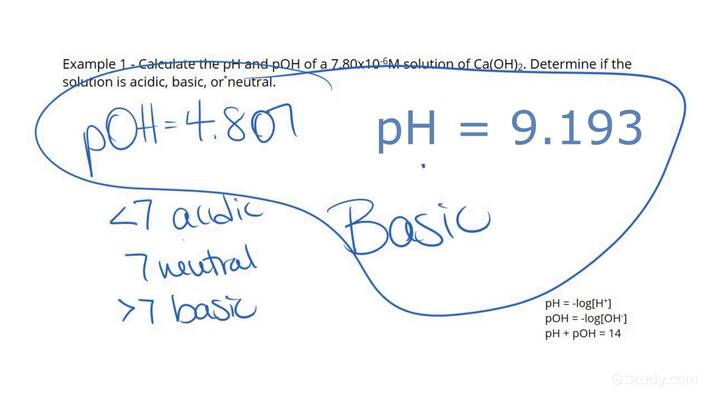
Understanding the formulas is key:
- pH = -log[H+] or -log[H3O+]
- pOH = -log[OH-]
- And importantly, pH + pOH = 14 in an aqueous solution at 25°C.
These formulas allow you to calculate pH or pOH if you know the concentration of hydronium ions (H3O+) or hydroxide ions (OH-) in a solution.
Utilize Logarithms for Precision

The logarithmic nature of pH and pOH scales makes precision crucial. Here’s how you can ensure accuracy:
- When calculating pH, you are finding the negative logarithm of the molar concentration of [H+].
- Similarly, for pOH, you’re calculating the negative logarithm of [OH-].
- Remember, log laws can help: log(a*b) = log(a) + log(b).
Consider Temperature’s Effect

The constants in pH and pOH calculations are temperature-dependent. Here are some considerations:
- The autoionization of water, or the equilibrium constant for the dissociation of water (Kw), changes with temperature.
- At 25°C, Kw = 1 x 10-14, but it shifts to different values at other temperatures. For example, at 0°C, Kw = 1.5 x 10-15.
- Note: For accurate pH and pOH calculations, know the temperature and use the appropriate Kw.
Practice with Problem-Solving Techniques

Applying the above knowledge requires practice. Here are some techniques to help you excel:
- Use dimensional analysis to ensure units are consistent.
- Check if the solution contains salts of weak acids or bases, as these might affect the pH or pOH through hydrolysis.
- Solve problems both algebraically and by using a calculator. It helps you verify your answer and learn from any discrepancies.
💡 Note: pH and pOH values are based on logarithms, which inherently involve precision. Always check your significant figures to ensure the accuracy of your calculations.
The mastery of pH and pOH calculations is not just about understanding chemical reactions or the acid-base balance. It's about applying this knowledge practically in various scientific fields. Remember the fundamental principles we've discussed, keep practicing, and always consider the nuances like temperature that can shift your calculations. With these tips, you're well on your way to achieving a deeper understanding and proficiency in acid-base chemistry.
What is the relationship between pH and pOH?
+
In an aqueous solution at 25°C, the sum of pH and pOH equals 14. This means pH + pOH = 14, which helps to interconvert pH and pOH values.
Why does temperature affect pH and pOH?
+
Temperature affects the autoionization of water, changing the value of the ion product of water, Kw. As Kw changes, so do the concentrations of H+ and OH- ions, hence altering pH and pOH.
Can I use pH paper to measure pH accurately?

+
pH paper provides a rough estimation of pH. For more precise measurements, use a pH meter or electronic pH sensor which can give readings to two decimal places.
How do I calculate pH if I know pOH?

+
To calculate pH when you know pOH, subtract pOH from 14. pH = 14 - pOH.
What happens when you add an acid or a base to a solution?

+
Adding an acid decreases the pH and increases the pOH, while adding a base increases the pH and decreases the pOH. The change depends on the amount of acid or base added and the buffering capacity of the solution.