Master Periodic Trends with Our Answer Key Guide

Periodic trends play a critical role in understanding the properties and behavior of elements in the periodic table. This guide aims to offer insights into the fundamental periodic trends that define how elements interact, react, and form bonds. With a systematic approach, we'll delve into atomic size, ionization energy, electron affinity, electronegativity, and metallic character, providing comprehensive explanations and leveraging our Answer Key Guide to solidify your understanding.
The Concept of Periodic Trends


The periodic table isn’t just an arrangement of elements; it’s a framework that reveals the intrinsic properties of atoms and their behavior. Periodic trends are recurrent changes in properties when moving across or down the periodic table:
- Atomic Size (atomic radius) indicates the size of an atom.
- Ionization Energy measures the energy required to remove an electron from an atom.
- Electron Affinity reflects the energy change when an atom gains an electron.
- Electronegativity is the measure of an atom’s ability to attract and hold onto electrons.
- Metallic Character describes how easily an atom can lose electrons to form a cation.
Atomic Size: Trends and Influences

Understanding atomic size involves knowing:
- Effective Nuclear Charge: The protons’ pull on the electrons, attracting them closer to the nucleus.
- Shielding Effect: The extent to which inner electrons shield outer electrons from the nuclear charge.
- Principal Energy Level (n): As atoms increase in energy level, the distance from the nucleus to the outermost electrons grows.
⚗️ Note: Across a period, atomic size decreases due to increasing effective nuclear charge pulling electrons closer. Down a group, atomic size increases as electrons are added to higher principal energy levels.
Unraveling Ionization Energy

Ionization energy trend:
- It generally increases from left to right across a period due to increasing effective nuclear charge, making electron removal more difficult.
- It decreases down a group because the outermost electrons are further from the nucleus, easing removal.
Electron Affinity: Gaining Insight

Electron affinity trends are not as straightforward but follow:
- In general, electron affinity increases (becomes more negative) across a period, as atoms want to achieve stability by gaining electrons.
- It tends to decrease down a group since larger atoms have less attraction for an added electron.
Electronegativity: Atoms’ Attraction

The electronegativity scale, as conceptualized by Linus Pauling:
- Electronegativity increases across a period due to the increased effective nuclear charge.
- Electronegativity generally decreases down a group because the attraction for electrons diminishes with the larger atomic size.
Metallic Character: Navigating Through Chemistry

Metallic character is determined by:
- How readily an atom loses electrons, decreasing across a period but increasing down a group.
- Elements to the left and bottom of the periodic table exhibit higher metallic character.
⚗️ Note: An atom's metallic character directly relates to its electron configuration, energy levels, and the electrons' ease of movement, known as delocalized electrons in metals.
Utilizing Periodic Trends for Problem Solving
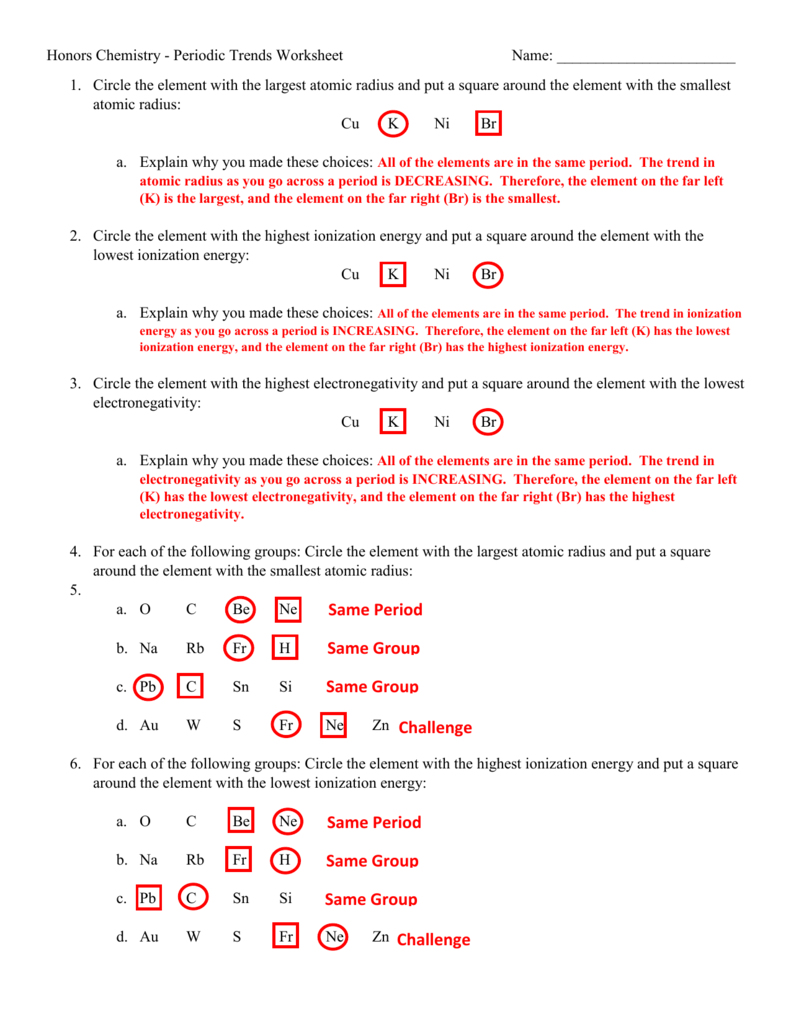
Mastering periodic trends allows for predictions in chemical reactions:
- Identify reactive elements, the likely oxidation states of ions, and which elements might form ionic or covalent bonds.
- Utilize trends to predict acid-base properties, solubility, and reactivity with other elements or compounds.
In Closing

Periodic trends are the backbone of understanding chemistry at its fundamental level. They allow us to predict the behavior of elements and compounds in chemical reactions, providing a roadmap to navigate the vastness of chemistry. By grasping these trends, you unlock a deeper comprehension of chemistry, from the simplest molecules to complex structures and reactions.
This guide has outlined key periodic trends, including atomic size, ionization energy, electron affinity, electronegativity, and metallic character, and provided insights into how these properties are interconnected. Applying these concepts will enhance your problem-solving skills in chemistry, enabling you to anticipate how elements will interact.
Why do ionization energies increase across a period?

+
As you move across a period from left to right, the effective nuclear charge increases, meaning the protons in the nucleus more strongly attract the electrons, requiring more energy to remove an electron from the atom.
How do periodic trends influence chemical bonding?

+
Periodic trends like electronegativity help predict the type of bond formed (ionic or covalent) and the polarity of those bonds. Elements with high electronegativity will pull electrons more strongly, leading to polar bonds or ionic bonds when the difference in electronegativity is significant.
Can periodic trends predict the reactivity of an element?

+
Yes, periodic trends can give insights into reactivity. For instance, elements at the bottom left of the periodic table (metals) have low ionization energies and are highly reactive since they easily lose electrons. Conversely, elements at the top right (nonmetals) gain electrons readily due to high electron affinity, making them also reactive.
How does atomic size affect the behavior of elements?

+
The size of an atom influences how closely other atoms can approach it during bonding and how it shares electrons. Smaller atoms with high effective nuclear charges form stronger bonds, while larger atoms with additional electron shells tend to form weaker bonds due to less effective nuclear charge.
What is the significance of knowing periodic trends?

+
Understanding periodic trends is crucial for predicting chemical behavior, understanding why elements combine in the manner they do, and for designing chemical reactions and syntheses. It forms the basis of chemical theory and has practical applications in all areas of science and industry.