Mastering the Periodic Table: Chemistry Worksheet Guide

Learning chemistry can be both an exhilarating and daunting experience, especially when it comes to understanding the cornerstone of chemical science: the Periodic Table. This guide aims to demystify the Periodic Table through a detailed exploration of its structure, elements, trends, and applications. Let's embark on this journey to make your chemistry homework not just easier, but also more fascinating.
Understanding the Structure of the Periodic Table
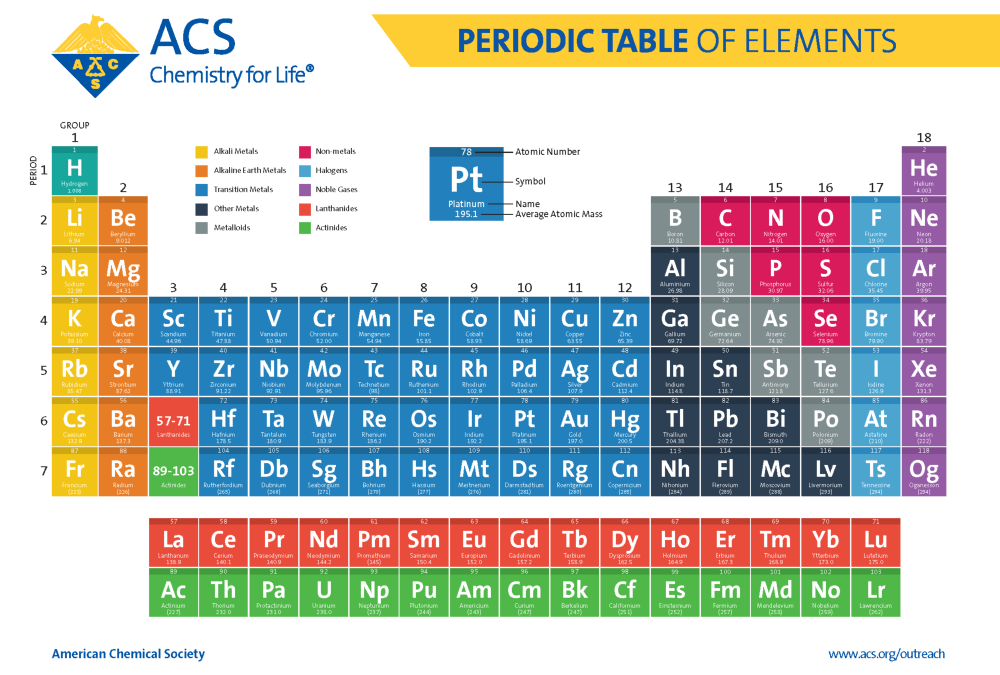
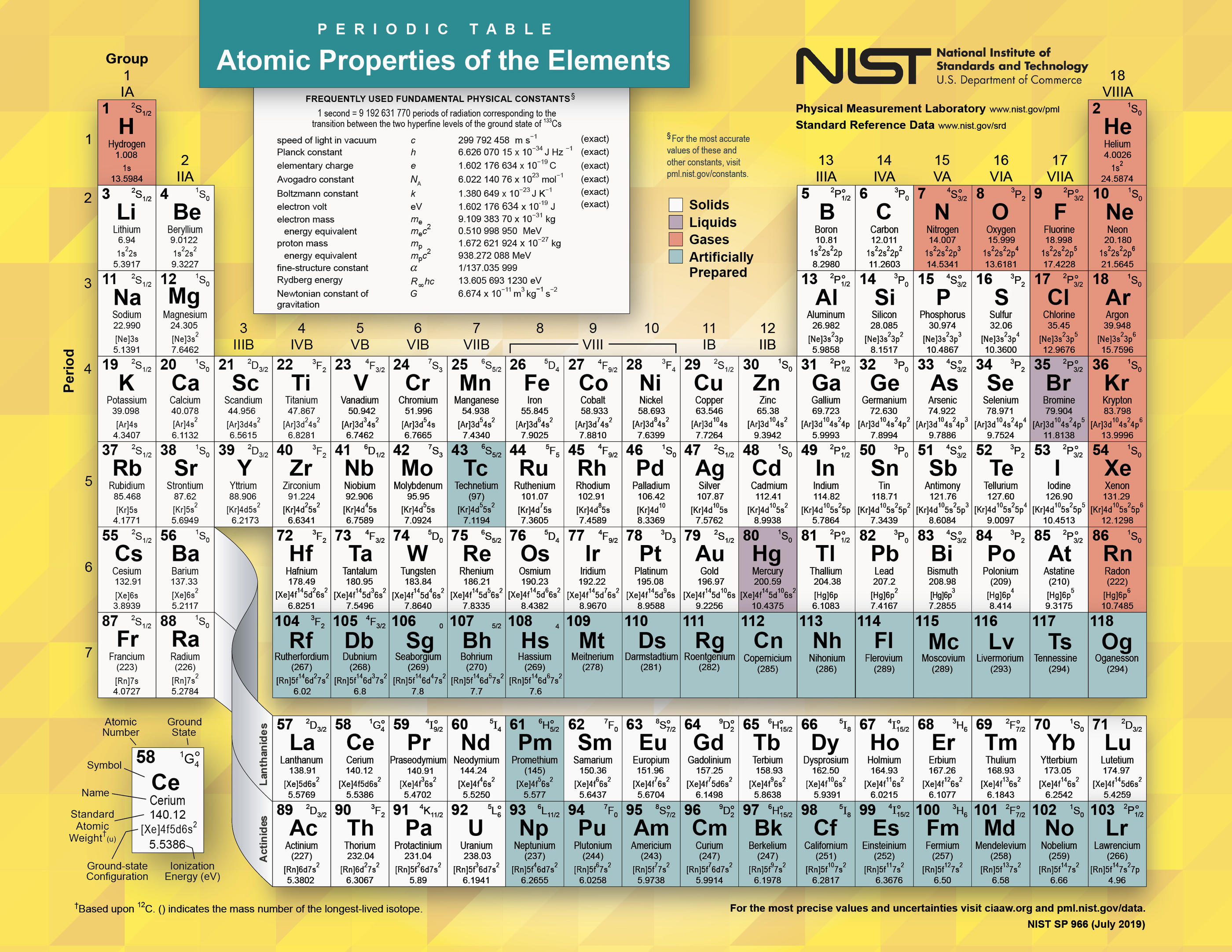
The Periodic Table is an organized chart where all known elements are listed in order of their atomic numbers, electron configurations, and recurring chemical properties. Here's a breakdown:
- Rows (Periods): Each row represents a period where elements have the same number of electron shells.
- Columns (Groups or Families): Elements in the same column share similar chemical properties because they have the same number of electrons in their outer shell, also known as valence electrons.
- Blocks: The table is divided into blocks based on the electron configurations:
- s-block: Groups 1 & 2, where the outermost electron(s) are in s-orbitals.
- p-block: Groups 13 to 18, with the outermost electron(s) in p-orbitals.
- d-block: Transition metals, which begin after Group 2 and span most of the center of the table.
- f-block: Often separated at the bottom of the table, these are the lanthanides and actinides, with electrons filling 4f and 5f orbitals respectively.
Element Classes on the Periodic Table

The elements can be broadly categorized into several classes:
- Metals: Found on the left side of the staircase line, metals are shiny, malleable, ductile, and good conductors of heat and electricity.
- Non-metals: On the right side, non-metals are generally dull, brittle, poor conductors, and exist in various forms (solid, liquid, gas).
- Metalloids: Elements along the staircase line that have properties intermediate between metals and non-metals.
- Noble Gases: Group 18, these gases are inert due to their full outer electron shells.
- Alkali Metals: Group 1, except hydrogen, very reactive.
- Alkaline Earth Metals: Group 2, also reactive but less so than alkali metals.
Key Trends and Properties

Understanding the trends across and down the Periodic Table is crucial for tackling chemistry problems:
Atomic Radius

- Across a Period: Atomic radius decreases as the effective nuclear charge increases, pulling electrons closer.
- Down a Group: Atomic radius increases as additional electron shells are added, increasing the distance from the nucleus to the outer electrons.
Ionization Energy

- Increases Across a Period: Due to increasing nuclear charge.
- Decreases Down a Group: Because the electrons are further from the nucleus, making it easier to remove an electron.
Electronegativity
- Increases Across a Period: More protons pull electrons more strongly.
- Decreases Down a Group: Electrons are at higher energy levels, reducing electronegativity.
Metallic Character
- Increases Down a Group: Elements become more metallic, as they can more easily lose electrons.
- Decreases Across a Period: As you move from left to right, elements become less metallic due to higher ionization energies.
Periodic Table in Chemistry Worksheets

When faced with chemistry worksheets, students often encounter questions that test their understanding of the Periodic Table:
- Identify Elements: Given an element's symbol, atomic number, or atomic mass, you must locate it on the Periodic Table.
- Element Properties: Questions might ask about an element's valence electrons, electron configuration, or expected chemical behavior based on its group.
- Periodic Trends: Understanding trends like electronegativity or ionization energy can help predict reactivity, bonding behavior, and other properties.
- Chemical Equations: Predicting reactions by understanding the element's placement in the Periodic Table.
To aid in these tasks, here's a handy table of common periodic trends:
| Trend | Across a Period | Down a Group |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Ionization Energy | Increases | Decreases |
| Electronegativity | Increases | Decreases |
| Metallic Character | Decreases | Increases |

🔍 Note: This table is a simplification; real trends might have exceptions due to factors like electron-electron repulsion or subshell energy differences.
Tips for Tackling Chemistry Worksheets

- Memorize Key Elements: Focus on the first 20 elements, transition metals, and some key non-metals. Knowing these will give you a solid foundation.
- Use Visual Mnemonics: Visual aids can help you remember the structure of the table and the trends more effectively.
- Understand Concepts, Not Just Memorize: While it's beneficial to know some facts by heart, understanding the logic behind the Periodic Table's layout is far more useful.
- Practice, Practice, Practice: The more you work with the Periodic Table, the more intuitive its use will become.
- Utilize Digital Tools: Apps or websites that allow interactive exploration of the Periodic Table can be invaluable for learning and reference.
As we conclude our comprehensive guide on mastering the Periodic Table through chemistry worksheets, it's clear that a deep understanding of this scientific tool is essential for success in chemistry. By grasping its structure, recognizing element classifications, and understanding periodic trends, students can tackle even the most complex questions with confidence. Remember, the journey through the Periodic Table is one of discovery, where each element tells its own story of atomic behavior and chemical interaction.
What is the significance of the Periodic Table’s groups?
+
Elements within the same group have similar properties because they have the same number of electrons in their outer shell, which influences their chemical reactivity and behavior in bonds.
How can I remember the Periodic Table?

+
Use mnemonics, acronyms, songs, or visual aids. Understanding the logic of why elements are placed where they are also helps in retention.
Why do some Periodic Tables have gaps?

+
Gaps represent elements that are yet to be discovered or synthesized. These gaps were initially predicted based on trends and later confirmed when new elements were discovered or created.
What does the atomic number represent?

+
The atomic number is the number of protons in the nucleus of an atom. It uniquely identifies an element and determines its place in the Periodic Table.
Can I use the Periodic Table to predict chemical reactions?

+
Yes, by understanding the electron configurations, you can predict how elements will react based on their tendency to gain, lose, or share electrons to achieve a stable electron configuration.