7 Must-Know Periodic Table Trends for Exams
The Periodic Table isn't just a collection of elements; it's a treasure map for understanding chemistry. Understanding its trends is critical for acing science exams, whether you're a high school student or preparing for advanced placement tests. Let's dive into the seven must-know periodic table trends that can give you an edge in your studies.
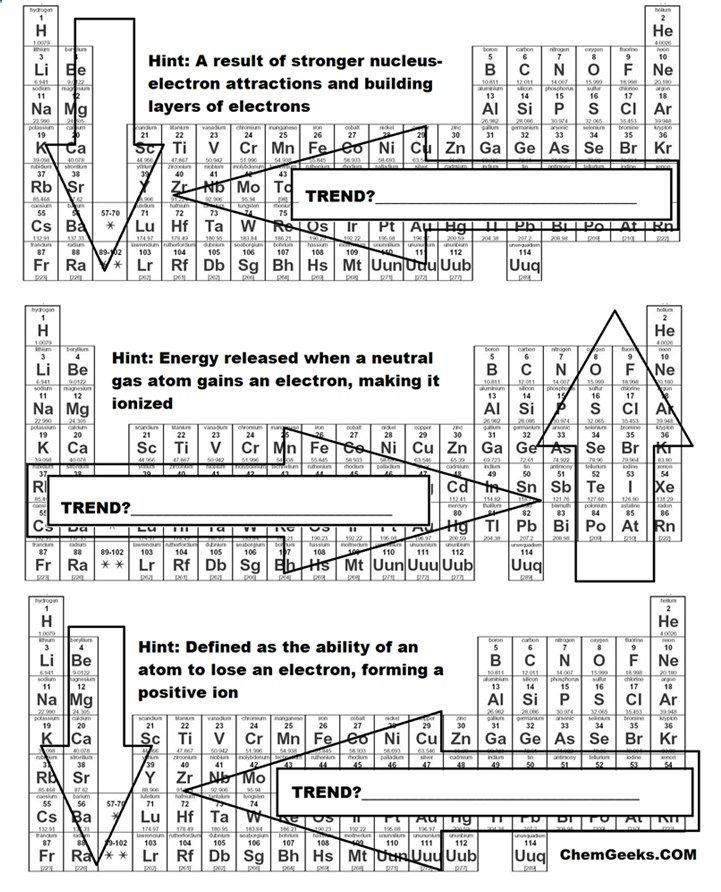
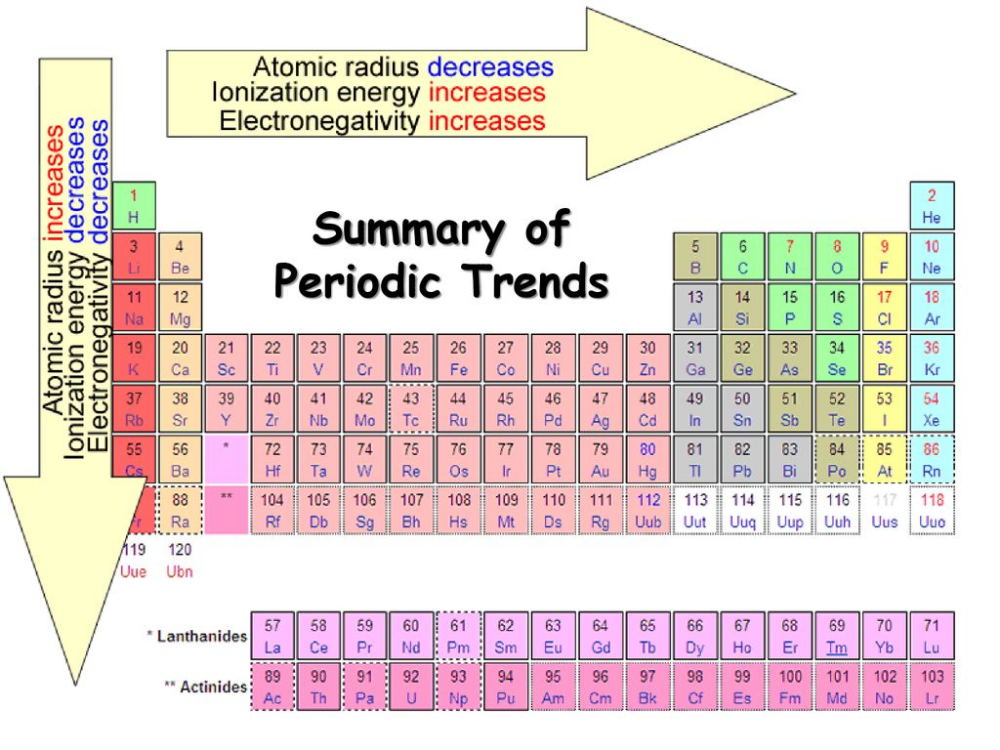
Atomic Radius

Atomic radius refers to the size of an atom, often measured by the distance from the nucleus to the outer electron shells:
- Down a Group: The atomic radius increases due to additional electron shells being added.
- Across a Period: The atomic radius decreases as the effective nuclear charge increases, pulling electrons closer to the nucleus.
It's worth noting that while atomic size trends have exceptions, these general patterns are reliable.
Ionic Radius
Ionic radius describes the size of an ion:
- Positive Ions (Cations): Losing electrons, they become smaller because the protons in the nucleus have less electrons to hold, tightening their orbit.
- Negative Ions (Anions): Gaining electrons, they expand due to increased electron-electron repulsion.
💡 Note: Be mindful that when atoms lose or gain electrons, they form ions which can significantly change their size compared to their neutral states.
Ionization Energy
This trend represents the energy required to remove an electron from an atom or ion:
- Down a Group: Ionization energy generally decreases due to the shielding effect of inner shells, making it easier to remove an outer electron.
- Across a Period: It increases as electrons are closer to the nucleus and more tightly held.
Electron Affinity

Electron affinity measures how eagerly an atom will accept an additional electron:
- Across a Period: Electron affinity increases towards the right, as elements become more non-metallic.
- Down a Group: Generally, it decreases because the added electron enters a new shell, further from the nucleus.
Electronegativity

Electronegativity describes an atom’s tendency to attract electrons in a covalent bond:
- Across a Period: Electronegativity increases due to increased nuclear charge attracting electrons more.
- Down a Group: It decreases because the valence electrons are farther from the nucleus, leading to reduced attraction.
💡 Note: Electronegativity scales like the Pauling scale are useful for understanding bond polarity.
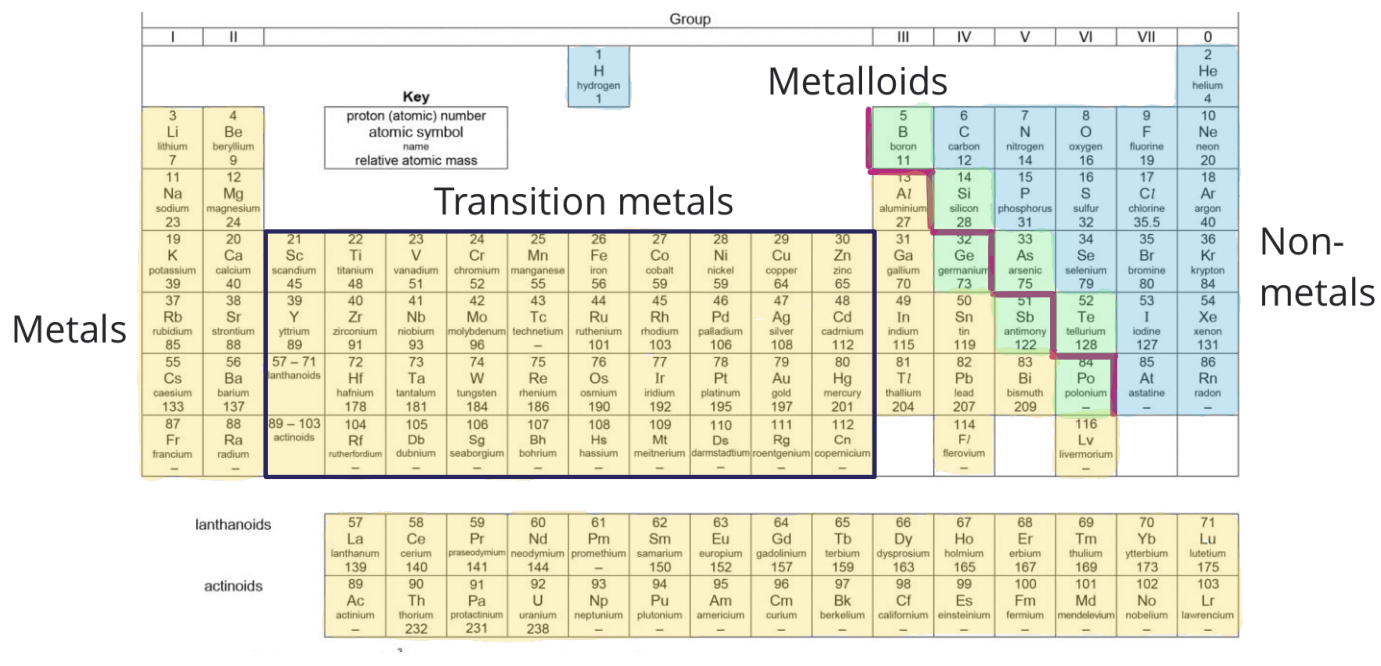
Metallic and Nonmetallic Character

This trend shows the nature of elements on the Periodic Table:
- Metals: Tend to be on the left side of the table, losing electrons easily, and have lower ionization energies.
- Non-metals: Found on the right, they gain electrons easily with high electron affinities and electronegativity.
- The transition occurs diagonally from left to right, with elements becoming more non-metallic.
Chemical Reactivity
How elements interact chemically varies:
- Metals: Reactivity increases as you go down a group due to easier electron loss.
- Non-metals: Reactivity decreases down a group but increases from left to right across a period.
Summing Up Key Points
Understanding these Periodic Table trends helps in predicting chemical behaviors, identifying unknown substances, and understanding why elements bond in specific ways. Keep in mind that these trends are general patterns, but there are exceptions due to factors like electron configuration. Knowing when and why these trends break down can also give you an insightful advantage in chemistry.
By applying these trends, you can make educated guesses about an element's properties, even without knowing its exact electron configuration or ionization energy. However, always remember to check experimental data when available.
In your studies, focus on:
- How these trends interrelate (e.g., high electronegativity often correlates with high ionization energy).
- Exceptions to the rule, like the d-block elements that often defy these trends.
- Using the Periodic Table's structure to visualize and understand these trends visually.
💡 Note: While these trends offer a roadmap, chemistry is full of surprises. Learning to recognize anomalies can distinguish a student from their peers.
This understanding not only aids in acing your exams but also forms the foundation for higher-level chemistry education and practical applications in various scientific fields.
Why do atomic radii increase down a group?

+
As you move down a group, each element has an additional electron shell which increases the distance between the nucleus and the outer electrons, thereby increasing atomic size.
How can understanding periodic trends help in predicting chemical behavior?
+
Periodic trends allow for predictions about reactivity, bond formation, and the types of compounds elements might form, based on properties like ionization energy and electronegativity.
What is the significance of the noble gases in terms of periodic trends?
+
Noble gases have the highest ionization energies and lowest electron affinities of their respective periods due to their full outer electron shells, making them inert under most conditions.