Mastering Chemistry: 5 Steps to Solve Percentage Composition Worksheet Answer Key

Percentage composition is a critical concept in chemistry that reveals how much each element contributes to a compound's mass. Whether you're a student trying to understand the basics or an enthusiast wanting to delve deeper into chemical analysis, mastering the calculation of percentage composition is fundamental. This guide offers a comprehensive walkthrough on how to tackle these calculations, providing a worksheet answer key to serve as both an educational tool and a reference.
Step 1: Identify the Molecular Formula

Before you dive into the calculations, ensure you have the molecular formula of the compound. This formula tells you which elements are present and in what ratio. Let’s take ethanol (C2H5OH) as an example:
- Carbon: 2 atoms
- Hydrogen: 6 atoms (5 from CH5 + 1 from OH)
- Oxygen: 1 atom
Step 2: Calculate the Molar Mass
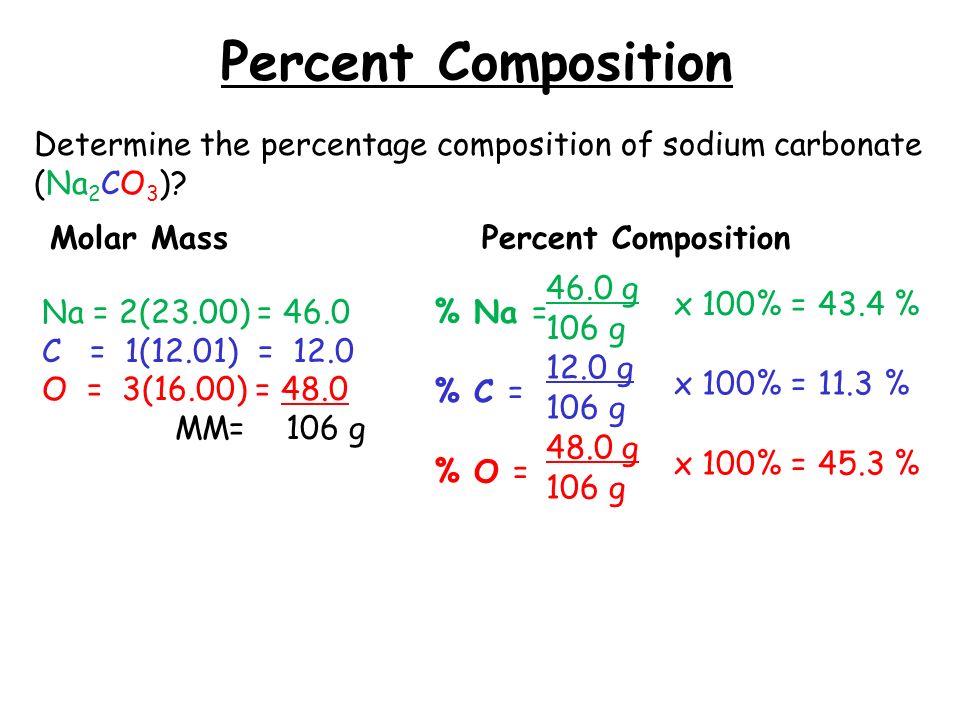
Next, you need to determine the molar mass of the entire compound and each element individually. Here’s how you do it:
| Element | Atomic Mass (u) | Number of Atoms | Contribution to Molar Mass (g/mol) |
|---|---|---|---|
| Carbon © | 12.01 | 2 | 24.02 |
| Hydrogen (H) | 1.008 | 6 | 6.048 |
| Oxygen (O) | 16.00 | 1 | 16.00 |
| Total Molar Mass | 46.068 | ||

The molar mass of ethanol is 46.068 g/mol.
Step 3: Determine the Mass Contribution of Each Element

Now, calculate the mass of each element in the compound using their atomic masses:
- Carbon: 2 atoms * 12.01 = 24.02 g
- Hydrogen: 6 atoms * 1.008 = 6.048 g
- Oxygen: 1 atom * 16.00 = 16.00 g
Step 4: Calculate the Percentage Composition

To find the percentage composition, divide the mass of each element by the total molar mass of the compound and multiply by 100:
- Percentage of Carbon = (24.02 / 46.068) * 100 = 52.1%
- Percentage of Hydrogen = (6.048 / 46.068) * 100 = 13.1%
- Percentage of Oxygen = (16.00 / 46.068) * 100 = 34.7%
Step 5: Verify Calculations

Always verify your calculations by ensuring that the total percentages add up to 100%. In our case:
52.1% + 13.1% + 34.7% = 100%
Remember that slight discrepancies might occur due to rounding, but they should be minor.
⚠️ Note: When dealing with rounding errors, ensure you use precise numbers and round at the end to avoid accumulating small errors.
The journey through chemistry's landscape requires understanding such fundamental concepts as percentage composition, which not only enriches your knowledge but also equips you to solve various problems in analytical and quantitative chemistry. By following these steps systematically, you can confidently approach any percentage composition problem with ease.
The key to mastering this calculation lies in understanding the relationship between the elements within a compound, their atomic masses, and how these contribute to the overall mass of the molecule. By applying this method, you'll be able to answer worksheet questions with precision, providing the basis for further exploration in chemistry.
Why do we need to calculate percentage composition?

+
Percentage composition helps chemists to understand the chemical identity of a compound, its purity, and how it might react in chemical reactions.
How accurate should my atomic mass values be?

+
The more precise the atomic masses you use, the more accurate your results. Most standard calculations use the atomic masses listed in periodic tables, which are often rounded to two decimal places.
Can I use the molecular weight instead of molar mass?

+
Yes, molecular weight and molar mass are essentially the same concept. However, ‘molar mass’ is the preferred term in modern chemistry, as it refers explicitly to the mass of one mole of a substance.