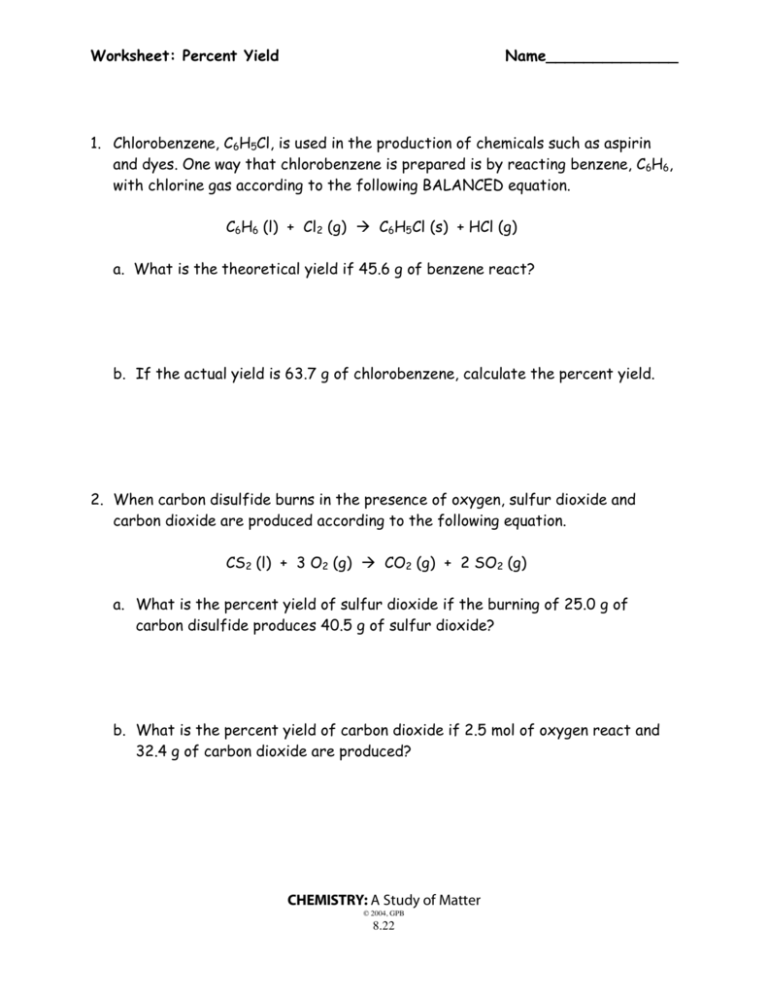
Master Percent Yield Calculations with This Worksheet

In the field of chemistry, the concept of percent yield is paramount for understanding the efficiency of chemical reactions. It's a measure that tells us how much of the desired product we can realistically expect, compared to the theoretical maximum. This insight is not just academic; it's crucial for optimizing industrial processes, controlling costs, and advancing research in both educational and professional settings. If you're looking to hone your skills in percent yield calculations, this comprehensive worksheet will guide you through the process with practical examples, detailed explanations, and useful tips.
Understanding Percent Yield

Percent yield calculation involves comparing the actual yield of a product from a reaction with the theoretical yield, which is the maximum amount of product that could be formed if the reaction were 100% efficient. Here's how you can calculate it:
- Actual Yield: The amount of product obtained from an experiment or process.
- Theoretical Yield: The calculated maximum amount of product that could be produced from the given amounts of reactants.
- Percent Yield Formula: Percent Yield = (Actual Yield / Theoretical Yield) * 100%
Worksheet: Steps to Calculate Percent Yield

1. Identify the Limiting Reagent
The limiting reagent is the substance that will be completely consumed first, thus determining the amount of product produced. Here’s how to find it:
- Write a balanced chemical equation for the reaction.
- Calculate the moles or mass of each reactant.
- Using the molar ratio from the balanced equation, determine which reactant will limit the reaction.
2. Calculate the Theoretical Yield

Based on the limiting reagent, calculate the theoretical yield:
- Convert the moles or mass of the limiting reagent to the moles or mass of the product using stoichiometry.
- Use the conversion factor from the balanced equation to get the theoretical yield in moles or mass.

3. Determine the Actual Yield

This is the yield you measure or obtain from your experiment or process. It’s what you physically collect.
4. Apply the Percent Yield Formula

Using the formula:
Percent Yield = (Actual Yield / Theoretical Yield) * 100%
5. Evaluate Your Results

- Calculate the percent yield to understand how efficient your reaction was.
- Analyze reasons for any discrepancy between theoretical and actual yields.
⚗️ Note: When calculating percent yield, remember that it's always less than or equal to 100%. A higher yield indicates a more efficient reaction, while a lower yield might suggest loss of product or side reactions.
| Reactant | Moles | Conversion Factor | Theoretical Yield (g) |
|---|---|---|---|
| Reactant A | 2 | 1:2 | 100 |
| Reactant B | 1.5 | 3:2 | 75 |

Practical Examples

Example 1: Combustion of Ethanol

In this example, we’ll calculate the percent yield for the combustion of ethanol to form water and carbon dioxide.
🔥 Note: This example assumes complete combustion of ethanol with no side products.
Example 2: Synthesis of Aspirin

Here, we’ll go through the synthesis of aspirin from salicylic acid, showcasing how to calculate percent yield in organic synthesis.
⚖️ Note: During synthesis, purity checks are crucial to ensure the product is what you expect.
The worksheet above provides a structured approach to mastering percent yield calculations. By following these steps and working through examples, you'll gain a deeper understanding of how theoretical and actual yields are related, and how to critically analyze your experimental results.
Why is percent yield always less than or equal to 100%?

+
Percent yield is always ≤ 100% because it represents how close you are to the theoretical maximum yield. Losses occur due to side reactions, inefficiencies, or incomplete reactions, making it nearly impossible to achieve 100% efficiency in a lab setting.
How can I improve my percent yield in experiments?

+
To improve percent yield, ensure:
- Optimal reaction conditions (temperature, pressure, catalysts).
- Accurate measurement of reactants.
- Minimal loss through spillage or evaporation.
- Effective recovery of product.
- Minimize side reactions with selective reagents or conditions.
What factors can lead to a lower percent yield?

+
Factors leading to a lower percent yield include:
- Incomplete reaction due to insufficient reaction time or suboptimal conditions.
- Loss during transfer or filtration of solids.
- Side reactions that produce by-products instead of the desired product.
- Errors in stoichiometry calculations.
- Purification steps that remove product along with impurities.