Atom Parts Worksheet Answers: Simplified Guide

In the world of chemistry and physics, understanding the basic building blocks of matter is crucial. The atom is the smallest unit of an element that retains the chemical properties of that element. Answering worksheets about atom parts can be a daunting task for students, but with a structured approach, this fundamental knowledge can be easily grasped. This guide will walk you through the primary components of an atom, their roles, and common misconceptions, helping you master your atom parts worksheet answers with confidence.
Understanding the Atom: Key Components

The atom consists of three fundamental particles:
- Protons: These are positively charged particles found in the nucleus of the atom. Their number defines the element’s atomic number and its identity.
- Electrons: These negatively charged particles orbit the nucleus in various energy levels or shells. Their behavior defines the atom’s chemical reactivity.
- Neutrons: These are neutral particles also found in the nucleus. Their presence contributes to the atom’s mass but does not affect its charge.
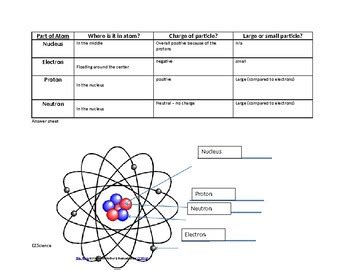
The Nucleus

The nucleus is the central part of an atom where protons and neutrons are housed. Here’s a quick breakdown:
| Particle | Charge | Mass | Location |
|---|---|---|---|
| Proton | +1 | 1 amu (atomic mass unit) | Nucleus |
| Neutron | 0 | 1 amu | Nucleus |
💡 Note: Although protons and neutrons both contribute to an atom’s mass, their numbers can differ, leading to isotopes of the same element.
Electron Shells

Electrons are arranged around the nucleus in specific energy levels, often visualized as shells:
- The first shell (closest to the nucleus) can hold up to 2 electrons.
- The second shell can hold up to 8 electrons, and so on, with higher shells following a formula based on the principal quantum number.
Atom Charge and Stability

When an atom has an equal number of protons and electrons, it is electrically neutral. If this balance is disturbed:
- An atom with more protons than electrons will have a positive ion or cation.
- An atom with more electrons than protons will have a negative ion or anion.
Common Misconceptions

Students often get confused by several key points:
- Mass of Electrons: Electrons are often overlooked due to their minuscule mass, but their positions and movements are vital in understanding an atom’s chemistry.
- Element vs. Isotope: The element is defined by its atomic number (number of protons), not the mass number (protons + neutrons). Thus, an isotope is an element with a different number of neutrons.
- Electron Orbitals vs. Shells: Orbitals are regions within shells where the probability of finding an electron is high, not exact paths like a planet orbiting a sun.
Working Through a Worksheet

When tackling an atom parts worksheet, follow these steps:
- Identify the atomic number to determine the element. This number matches the number of protons.
- Calculate the number of neutrons by subtracting the atomic number from the mass number (if given).
- Determine the number of electrons based on whether the atom is neutral or charged.
- Consider the electron configuration to understand how electrons are distributed in shells.
🧪 Note: Sometimes, worksheets might not give you all the information at once. You'll need to use the periodic table to deduce missing details.
In summary, mastering atom parts involves understanding the fundamental particles, their locations, and how they interact. This knowledge not only helps in answering worksheets but also in comprehending how chemical reactions occur and why elements behave as they do. By familiarizing yourself with these key concepts, you'll find that answering atom parts worksheets becomes second nature. Remember, atoms are the building blocks of everything around us, and understanding their structure is pivotal to science and technology.
What’s the difference between an atom and an ion?
+
An atom has an equal number of protons and electrons, making it neutral. An ion, however, has an imbalance, either having more protons (cation, positive ion) or electrons (anion, negative ion) than electrons.
How do electrons move around the nucleus?

+
Electrons don’t move in fixed orbits but exist in probability clouds called orbitals, where there is a higher chance of finding an electron.
Why are some isotopes radioactive?

+
Isotopes become radioactive when their nucleus has an unstable arrangement of protons and neutrons, which leads to decay processes to achieve stability.



