5 Ways to Understand Parts of an Atom Easily

Delving into the structure of the atom, one of the most fundamental entities of nature, can be both fascinating and complex. Understanding the atomic parts can demystify various phenomena in science and provide a clearer comprehension of how matter behaves. Here are five effective methods to grasp the concept of parts of an atom with ease:
1. Visual Aids and Atomic Models


Using visual tools is an impactful way to understand the components of an atom:
- Bohr Model: A simplified model of the atom where electrons orbit the nucleus in fixed paths like planets around the sun.
- Cloud Model: This represents electron behavior as a probability cloud rather than specific orbits, indicating where electrons are most likely to be found.
- Quantum Model: A more complex visualization showing electrons in atomic orbitals, their shape, and energy levels.
📌 Note: Each model has its limitations but they collectively help visualize the complex nature of atomic structure.
2. Interactive Simulations
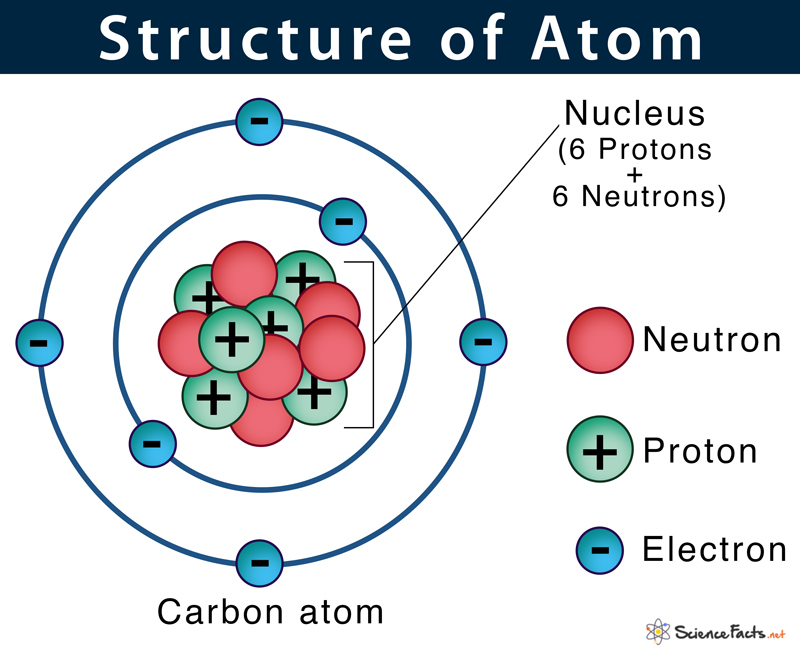
Interactive learning tools can greatly enhance your understanding:
- PhET Interactive Simulations: Developed by the University of Colorado Boulder, these simulations allow you to manipulate atoms and observe the effects on electron arrangements.
- Virtual Labs: Online platforms where students can conduct virtual experiments on atomic structure.
Interactive tools provide a dynamic learning environment, allowing for experimentation and immediate feedback, which fosters a deeper understanding.
3. Relating to Everyday Analogies
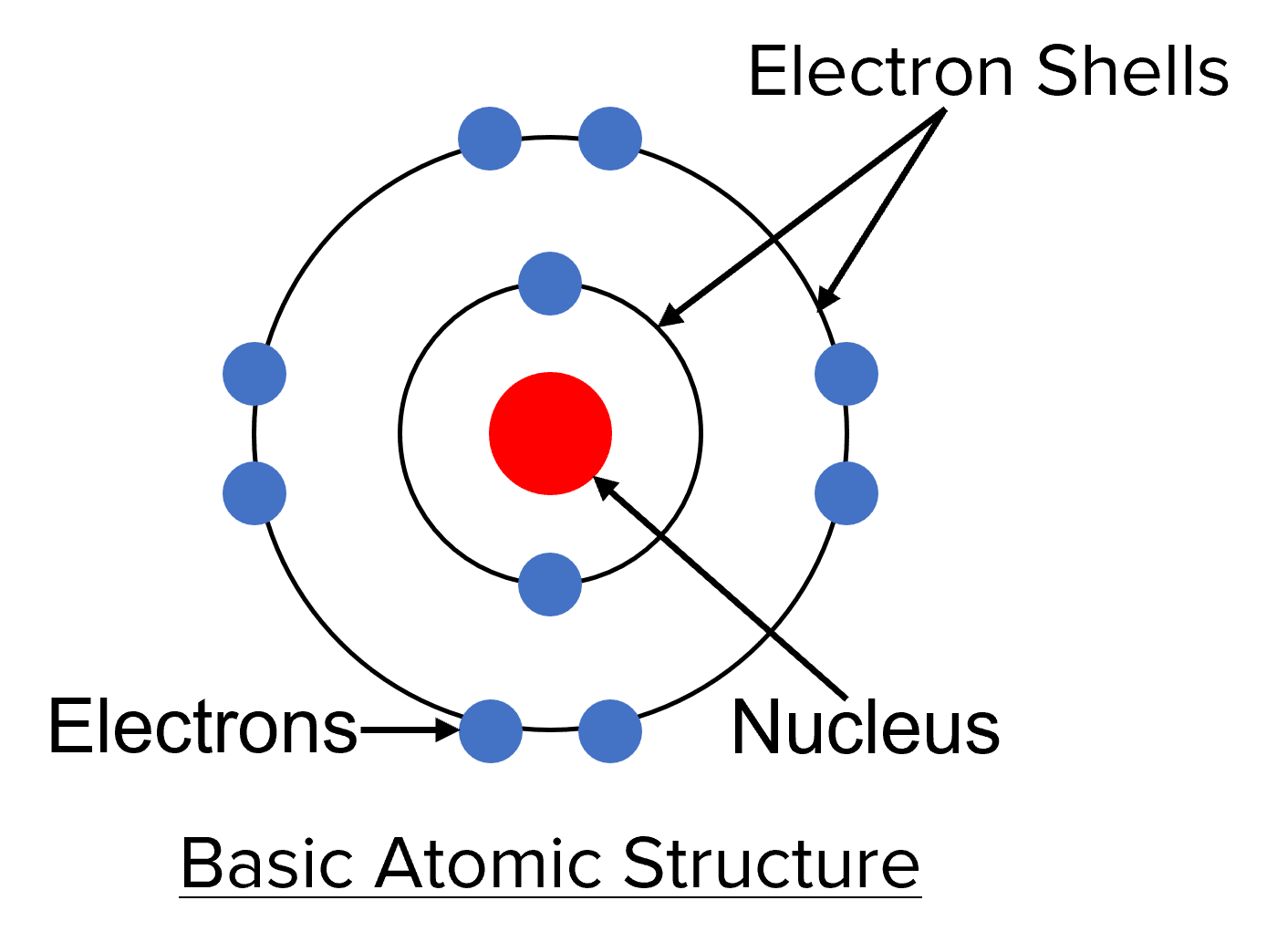
Analogies help in making abstract concepts tangible:
- The Solar System: The atom can be compared to the solar system, with the nucleus as the sun and electrons as planets.
- A Stadium: Imagine electrons as fans filling up different sections (energy levels) in a stadium, with the higher seats corresponding to higher energy levels.
- Bubbles in Water: Electrons can be seen as bubbles in water, where the water level represents energy levels and the bubbles are distributed around a central point (nucleus).
4. Engaging with Historical Perspectives


Understanding how our knowledge of the atom evolved helps contextualize its components:
- Democritus: Proposed that matter was made up of indivisible units called atoms.
- John Dalton: His atomic theory established the foundation for atomic studies, suggesting atoms could combine in simple ratios to form compounds.
- J.J. Thomson: Discovered the electron, leading to the “plum pudding” model.
- Ernest Rutherford: His gold foil experiment gave us the nuclear model of the atom.
Tracing the history of atomic theory provides insight into how our understanding of atoms was built up over time.
5. Experimentation at Home
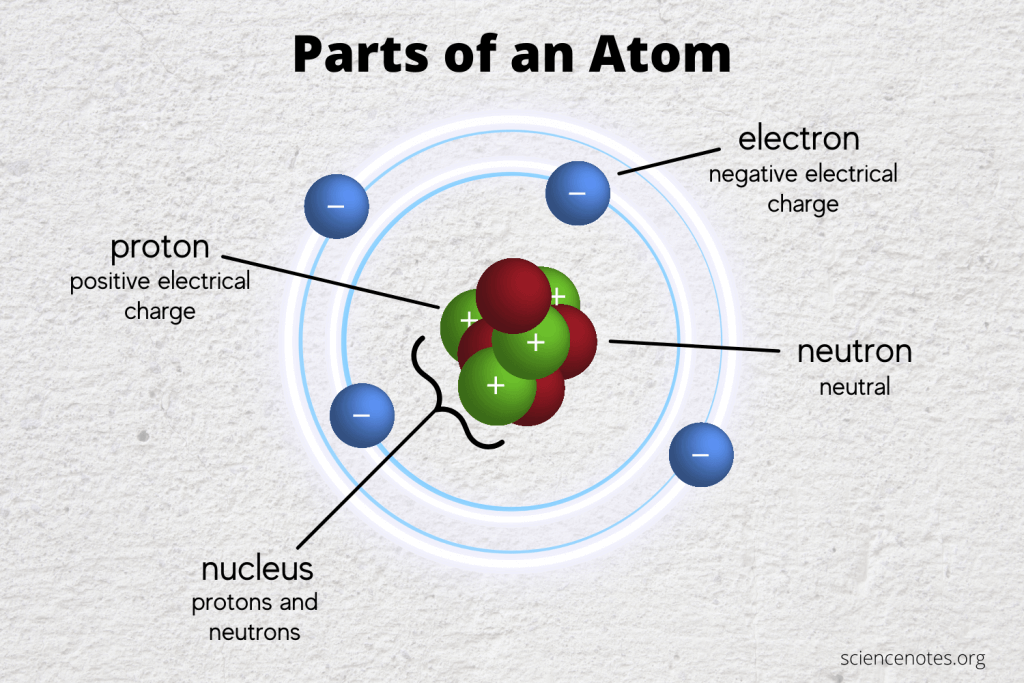
Sometimes, the best way to understand an atom is through hands-on activities:
- Build Your Own Atom: Use beads or other small objects to represent protons, neutrons, and electrons, and arrange them to form different atoms.
- Static Electricity Experiments: Observe how materials gain or lose electrons when rubbed, demonstrating the idea of electron transfer in atoms.
- Light Emission: Set up experiments with neon lights or other gas tubes to see how atoms emit light when excited.
These home experiments allow you to physically interact with the concepts of atomic structure, providing a direct link to theoretical knowledge.
As we wrap up our exploration of how to understand parts of an atom, it's evident that multiple approaches can enhance comprehension. Each method offers unique insights, from visualizing the atom through models to engaging with its historical development. By combining visual learning, interactive simulations, analogies, historical context, and practical experiments, you'll find the structure of an atom not only accessible but also captivating. The key takeaway is that atoms are dynamic entities, constantly in motion and interacting with their environment, a fact that's essential for grasping the fundamental nature of our world.
What is the nucleus of an atom?

+
The nucleus is the central core of an atom, which contains protons and neutrons. It carries a positive charge due to the protons.
Why do electrons orbit the nucleus?

+
Electrons orbit the nucleus because of the electromagnetic force. They are attracted to the positively charged nucleus and repel each other, which results in a stable orbit.
Can atoms be created or destroyed?

+
In chemical reactions, atoms are neither created nor destroyed, only rearranged. However, in nuclear reactions, atoms can be transformed through processes like fusion or fission.
How do electrons determine an atom’s chemical behavior?

+
Electrons, especially those in the outermost shell (valence electrons), determine an atom’s reactivity by either gaining, losing, or sharing electrons to achieve a more stable electronic configuration.
What are isotopes, and how do they relate to atoms?

+
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. They have similar chemical properties but different nuclear stability and mass.