Master Oxidative Phosphorylation with This Simple Worksheet

Understanding Oxidative Phosphorylation

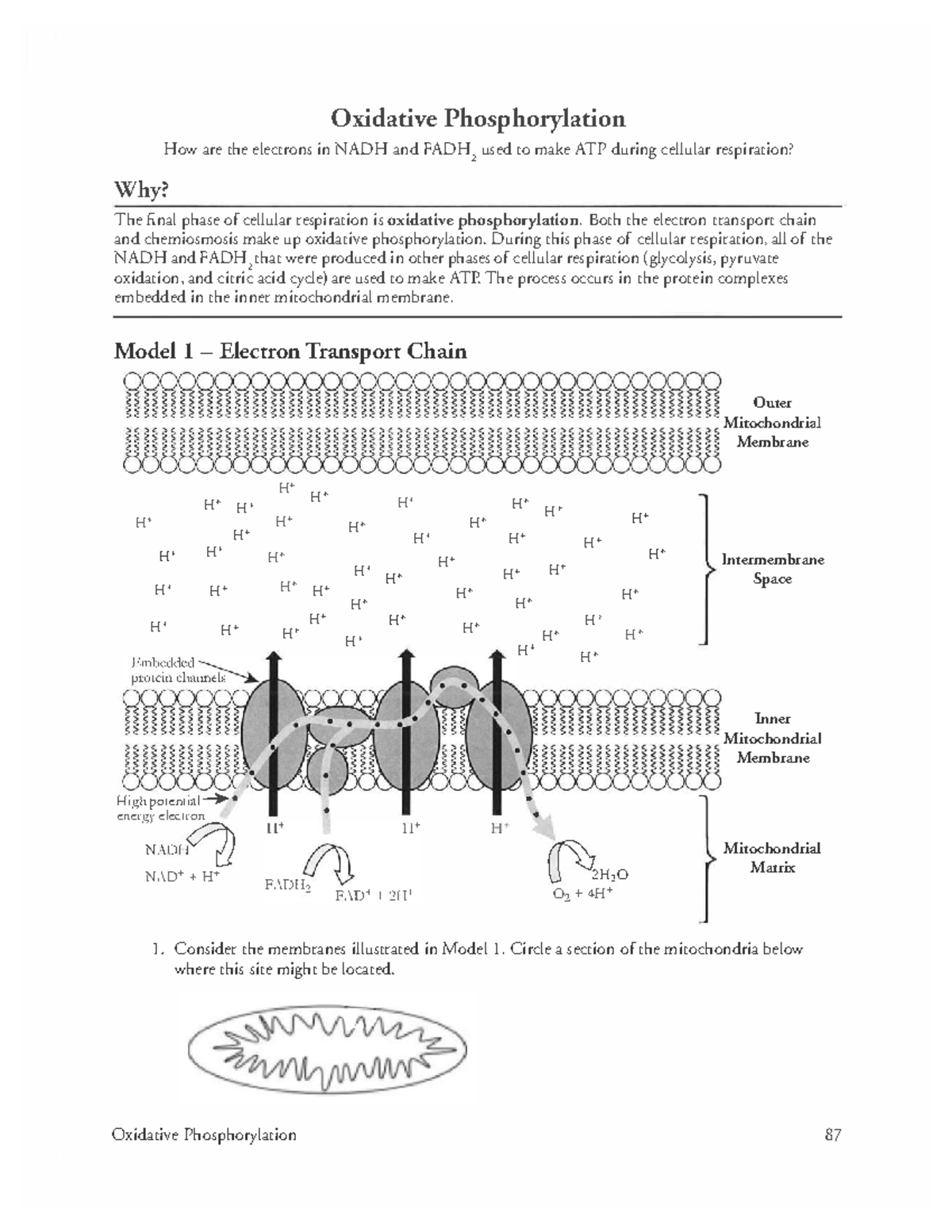
Oxidative phosphorylation is a crucial process in the electron transport chain that powers the synthesis of ATP in mitochondria. This process occurs in the inner mitochondrial membrane and involves the transfer of electrons through a series of protein complexes, leading to the creation of a proton gradient that drives ATP synthesis. Here’s a breakdown to master oxidative phosphorylation:
Steps of Oxidative Phosphorylation

1. Electron Transport Chain (ETC)

The electron transport chain is a sequence of protein complexes in the mitochondrial membrane. Here’s how it works:
- Complex I (NADH Dehydrogenase): Electrons from NADH enter the ETC. As electrons move from NADH to ubiquinone (CoQ), they pump protons into the intermembrane space.
- Complex II (Succinate Dehydrogenase): This complex directly receives electrons from succinate in the TCA cycle, but does not pump protons across the membrane.
- Complex III (Cytochrome bc1 Complex): Electrons from CoQ are transferred to cytochrome c, with additional proton pumping occurring.
- Complex IV (Cytochrome c Oxidase): Electrons finally reach oxygen to form water, further pumping protons.
2. Proton Gradient and Chemiosmosis

As protons are pumped across the mitochondrial membrane, a concentration gradient of protons (proton motive force) is created. This:
- Drives protons back into the mitochondrial matrix through the ATP synthase complex.
- This movement back into the matrix through ATP synthase provides the energy to phosphorylate ADP to ATP.
⚠️ Note: The proton motive force is essentially an electrochemical gradient that facilitates ATP production.
3. ATP Synthase

ATP synthase is the enzyme responsible for:
- Phosphorylation of ADP to ATP: It uses the proton gradient to drive ATP synthesis through chemiosmosis.
- Rotational Mechanism: Protons flowing through ATP synthase cause its rotor part to spin, which triggers ATP synthesis.
4. The Role of Oxygen

Oxygen serves as the final electron acceptor in the chain:
- It combines with electrons and hydrogen ions to form water, maintaining the ETC’s functionality.
- This ensures that electrons from NADH and FADH2 are continuously recycled back to their reduced forms.
5. Energy Yield
The overall yield of ATP from oxidative phosphorylation can be summarized:
| NADH | FADH2 | Approximate ATP Produced |
|---|---|---|
| Each NADH | - | 2.5 ATP |
| - | Each FADH2 | 1.5 ATP |

This table accounts for protons lost due to proton leak or other factors, which slightly reduces the theoretical maximum yield.
Understanding Efficiency

Oxidative phosphorylation is highly efficient compared to other forms of ATP production. Its efficiency stems from:
- The conversion of food energy into ATP through a controlled process that minimizes energy loss.
- A high potential yield, although actual ATP production can be influenced by numerous cellular factors.
In wrapping up, oxidative phosphorylation is an elegant and vital metabolic process that captures the energy released from food molecules to generate ATP. It showcases the complexity of cellular respiration and the intricate mechanisms cells use to convert energy. Remember, this process not only provides the bulk of ATP necessary for cellular functions but also underscores the critical role of mitochondria in our survival.
What is the primary function of the electron transport chain?

+
The primary function of the electron transport chain is to transfer electrons from NADH and FADH2 to oxygen, creating a proton gradient that drives ATP synthesis.
Why is oxygen necessary for oxidative phosphorylation?

+
Oxygen acts as the final electron acceptor in the ETC, which allows for the recycling of electron carriers and the creation of water as a byproduct.
How many ATP molecules are typically produced by one molecule of glucose?

+
In eukaryotic cells, one glucose molecule can yield up to about 30-32 ATP molecules via oxidative phosphorylation, considering the inefficiencies in the process.



