5 Tips for Mastering Oxidation State Worksheets

In the realm of chemistry, oxidation states play a pivotal role in understanding how atoms and ions interact within compounds. Grasping the nuances of oxidation states is not only fundamental for students but also for professionals in the field to comprehend chemical reactions, redox processes, and electron transfer mechanisms. Here are five essential tips for mastering oxidation state worksheets, aimed at ensuring you can conquer this complex topic with confidence and ease.
Understand the Basics

The foundation of mastering oxidation state worksheets lies in understanding what oxidation states are and why they matter. Atoms in compounds or ions can have different numbers of valence electrons, thus they exhibit different charges known as oxidation states. Here’s what you need to know:
- The oxidation state of an element in its standard state (like O2, H2) is zero.
- Oxygen typically has an oxidation state of -2, except in peroxides where it is -1.
- Hydrogen has an oxidation state of +1 in most compounds, except in metal hydrides where it is -1.
Remember, these are generalizations; there are exceptions due to electron sharing and bonding peculiarities.
💡 Note: Knowing these rules will help you quickly identify the oxidation states of elements in common compounds.
Practice Predicting Changes

Chemical reactions, particularly redox reactions, involve changes in oxidation states. Here are steps to practice predicting these changes:
- Identify which element undergoes oxidation (loses electrons) and which one reduction (gains electrons).
- Use the equation:
Element Initial State Final State Change Hydrogen (in HCl) +1 0 (as gas) +1 Iron (in FeCl3) +3 +2 (in FeCl2) -1 
- Verify the total change in oxidation states balances out for the entire reaction.
🔍 Note: Practice is key. The more reactions you analyze, the better you'll become at predicting oxidation state changes.
Learn Exceptional Cases

While the basic rules are critical, real-world chemistry often presents anomalies. Here are some exceptions to be aware of:
- Chlorine can have positive oxidation states like +1 in HOCl (hypochlorous acid) and +7 in ClO4- (perchlorate).
- Transition metals can exhibit multiple oxidation states due to their d-orbitals.
- Elements in lower groups of the periodic table can have negative oxidation states in some compounds.
Familiarity with these exceptions will prevent confusion when encountering unexpected oxidation states in chemical literature or lab work.
Utilize Reference Materials
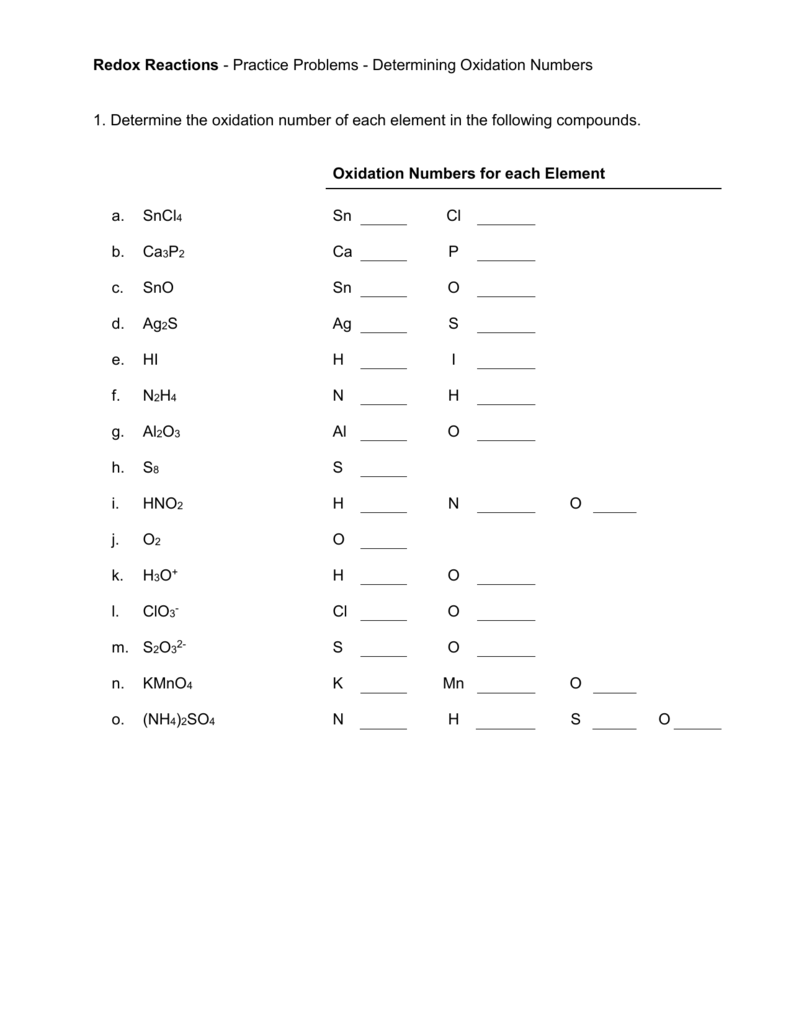
When faced with complex compounds or uncertain about oxidation states, reference materials can be invaluable. Here are some strategies:
- Consult a periodic table or an oxidation state chart for quick reference.
- Refer to textbooks or online resources like PubChem or ChembK for compound-specific data.
- Use mnemonic devices or tables summarizing common oxidation states for elements.
Remember, there's no need to memorize every single oxidation state. Knowing where to look for accurate information is just as important.
📚 Note: Keep reliable sources handy, and don’t be afraid to consult them during your work.
Balance Redox Equations

Balancing redox equations requires understanding oxidation states to track the electron transfer. Follow these steps:
- Assign oxidation states to all elements in the reactants and products.
- Identify the substances undergoing oxidation and reduction.
- Write half-equations for oxidation and reduction, balancing atoms other than O and H, then add electrons to balance charges.
- Balance electrons by multiplying equations with appropriate factors.
- Combine the half-equations, cancel out spectator species, and balance the rest of the equation.
By practicing this skill, you'll enhance your understanding of redox processes and oxidation state changes.
In summary, mastering oxidation state worksheets is about understanding fundamental principles, recognizing patterns, and practicing with real-world reactions. Utilizing reference materials, learning exceptions, and focusing on balancing redox equations will elevate your proficiency in this critical area of chemistry. Remember, persistence in practice and continuous learning are the keys to success.
What is an oxidation state?

+
Oxidation state, also known as oxidation number, is the theoretical charge that an atom in a molecule or ion would have if electrons were completely transferred from the less electronegative atom to the more electronegative atom.
How do I find the oxidation state of an element in a compound?

+
Use the basic rules such as knowing that the oxidation state of hydrogen in most compounds is +1, oxygen is typically -2, and sum the oxidation states to equal the charge of the ion or the net charge of the compound. Practice identifying these states by working through worksheets and reference materials.
Why are there exceptions to the oxidation state rules?

+
Exceptions occur due to complex electron sharing or bonding situations where the distribution of electrons doesn’t follow simple integer patterns. This is common with transition metals, where electron d-orbitals allow for variable bonding behavior.



