5 Essential Tips for Osmosis Worksheet Answers
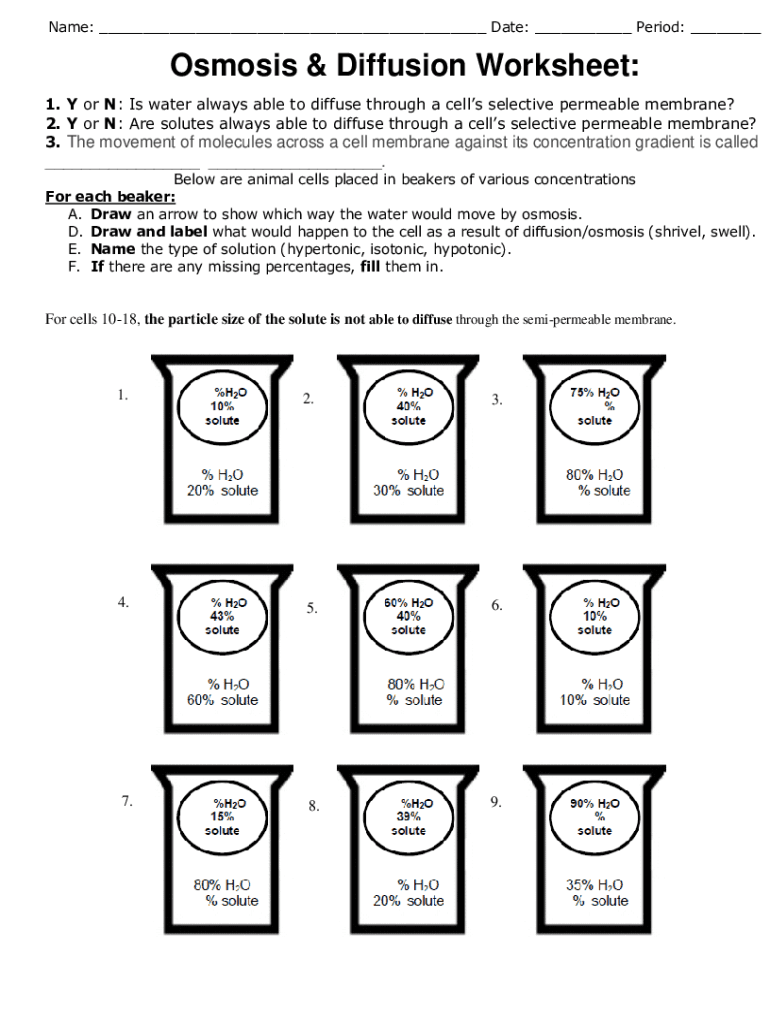
Enhancing Your Learning Through Effective Osmosis Worksheet Completion

Osmosis worksheets play a crucial role in understanding fundamental scientific concepts, particularly within the realm of biology, chemistry, and environmental science. These educational tools not only aid in grasping the mechanics of osmosis but also in developing critical thinking and problem-solving skills. Here are five essential tips to maximize the benefits of osmosis worksheet answers:
1. Understand the Basics of Osmosis
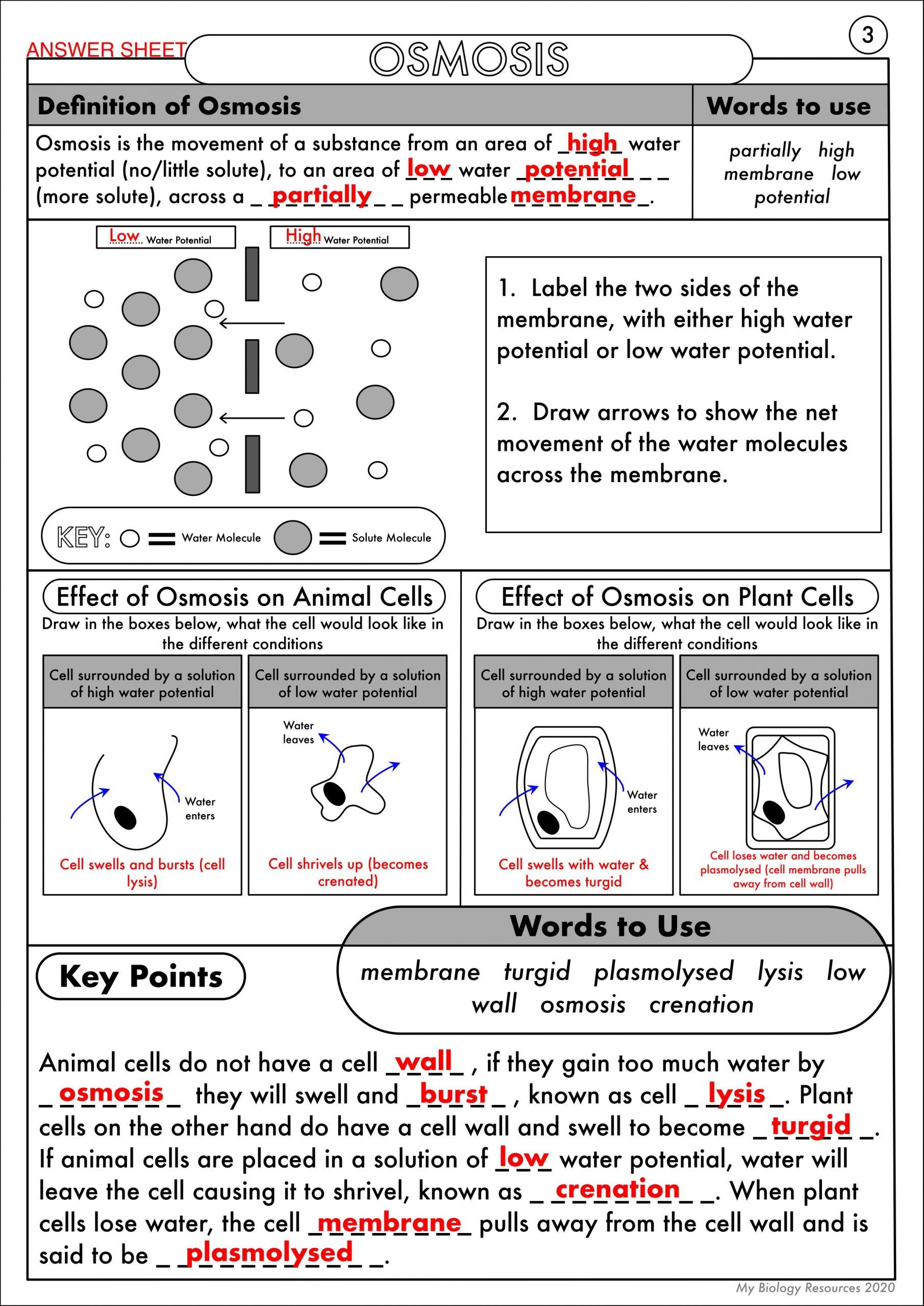
Before diving into osmosis worksheets, make sure you have a solid grasp of what osmosis actually is:
- Definition: Osmosis is the movement of solvent molecules (usually water) through a semipermeable membrane, from an area of higher solvent concentration to an area of lower concentration.
- Concepts to Know: Hypotonic, hypertonic, isotonic solutions; osmotic pressure; solute concentration; semipermeable membranes.

Understanding these basics will make interpreting the worksheet answers more intuitive.
2. Read Through the Worksheet Questions Carefully

Attention to detail is crucial when answering osmosis worksheets:
- Read each question thoroughly to understand what is being asked.
- Look for keywords like increase, decrease, or constant that indicate changes or lack thereof in solutions or pressures.
3. Apply Osmotic Potential Calculations

When dealing with osmosis, calculating the osmotic potential can be key:
| Formula | Description |
|---|---|
| ψ = ψs + ψp | ψ is the water potential, ψs is the solute potential, ψp is the pressure potential |
| ψs = -iCRT | i is the ionization constant, C is the molar concentration, R is the ideal gas constant, T is the absolute temperature |

Knowing these formulas can provide a quantitative approach to answering osmosis-related questions.
4. Utilize Diagrams and Visual Aids

Diagrams can significantly enhance understanding:
- Draw the process of osmosis before and after the event.
- Include arrows to represent the direction of water movement and solute concentration changes.

Visual aids will not only help with understanding but also in correctly answering related worksheet questions.
5. Review Your Answers and Check for Consistency

After completing your worksheet:
- Go over each answer to ensure they align with your understanding of osmosis.
- Check for consistency in your reasoning and answers.
Ensuring your answers make sense within the context of osmosis can boost confidence in your understanding.
💡 Note: Remember, osmosis worksheets are designed to help you learn and reinforce key concepts. Therefore, the learning process itself, rather than merely getting the right answers, is the true objective.
Working through osmosis worksheets effectively involves understanding the principles, reading carefully, applying formulas, visualizing the process, and reviewing your answers for consistency. By adhering to these tips, you can elevate your learning experience, ensuring a deeper comprehension of osmosis and its practical applications in various scientific fields. Remember, osmosis isn't just about water; it's a gateway to understanding cellular function, plant biology, and environmental interactions. By mastering these worksheets, you're not only preparing for exams but also building a solid foundation for further scientific inquiry.
What is osmosis?

+
Osmosis is the process where solvent molecules, typically water, move through a semipermeable membrane from a region of higher solvent concentration to a region of lower concentration. This movement aims to equalize the concentrations on both sides of the membrane.
Why is it important to understand osmosis?

+
Understanding osmosis is essential in biology as it underpins many physiological processes, including how cells maintain their shape and function, how plants absorb water, and how nutrients are transported within organisms. It also has applications in medicine, agriculture, and environmental science.
What is the difference between a hypotonic and hypertonic solution?

+
A hypotonic solution has a lower solute concentration than the cell, meaning water tends to move into the cell, potentially causing it to swell or burst. A hypertonic solution has a higher solute concentration than the cell, leading water to move out of the cell, which can cause it to shrink.
How do osmosis worksheets help in learning?

+
Osmosis worksheets provide practical exercises to reinforce theoretical knowledge. They encourage problem-solving, critical thinking, and the application of concepts in real-world scenarios, aiding in a deeper understanding of the topic.
Can osmosis principles be applied outside biology?

+
Absolutely! Osmosis principles are applicable in fields like agriculture for irrigation and fertilization, in medicine for understanding body fluid dynamics, and in environmental science for understanding water movement in ecosystems.