Osmosis and Tonicity Worksheet Answers: Master Biology Easily

Biology, at times, can be a complex maze of concepts that often require a great deal of memorization and understanding. Among these myriad concepts, osmosis and tonicity stand out as fundamental to cellular life. Whether you're a high school student cramming for a biology exam or a biology enthusiast keen on mastering the subject, this osmosis and tonicity worksheet guide will clarify these essential biological processes and aid your comprehension of cell behavior in different environments.
Understanding Osmosis

Osmosis is the passive movement of water molecules across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. The objective is to equalize the solute concentration on both sides of the membrane, although the net movement will only stop when an equilibrium is reached.
Key Factors of Osmosis

- The concentration gradient: Water moves from the hypotonic (low solute concentration) to the hypertonic solution (high solute concentration).
- Selective permeability of the membrane which allows passage of water but not of most solutes.
- Water molecules move passively, which means no energy is used by the cell.
Tonicity Explained

Tonicity describes how the concentration of solutes influences the direction of osmosis across a cell membrane. The term refers to the effect that the tonicity of the extracellular fluid has on the shape of the cell.
Types of Solutions Based on Tonicity
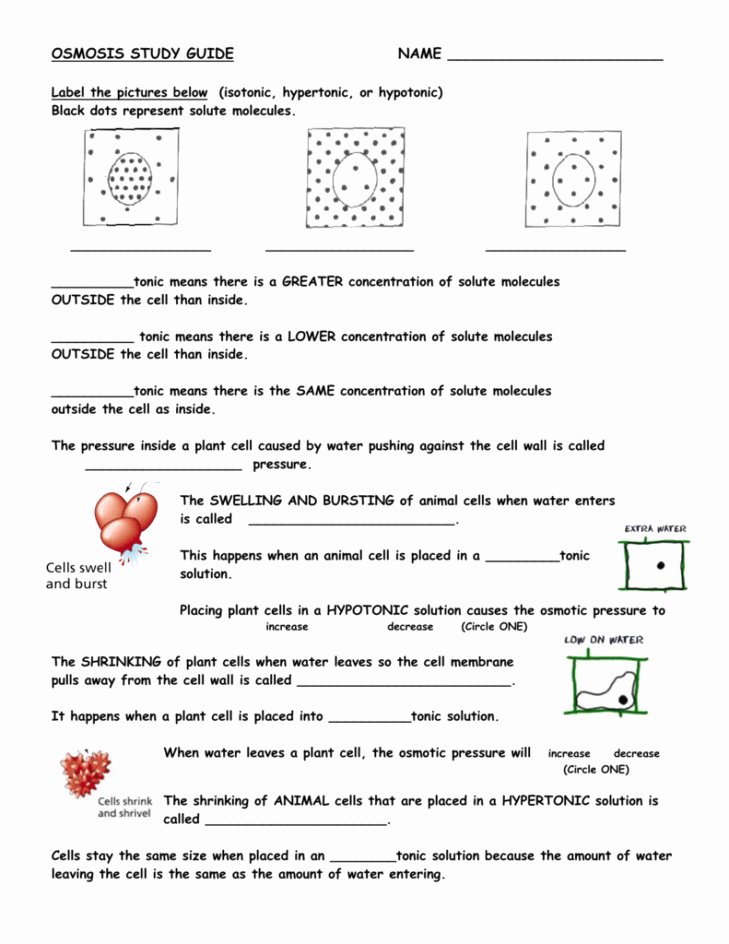
- Isotonic Solution: The solute concentration is the same on both sides of the membrane. No net movement of water, so the cell volume remains stable.
- Hypotonic Solution: The extracellular environment has a lower solute concentration than the cell’s interior. This leads to water influx into the cell, causing it to swell or potentially burst (lyse).
- Hypertonic Solution: Here, the external environment has a higher solute concentration, causing water to leave the cell. This can lead to shrinkage or crenation of the cell.
Worksheet on Osmosis and Tonicity

Below are some worksheet questions with their answers that can help you understand these concepts better:
Question 1: Explain Osmosis in a Plant Cell

Osmosis in a plant cell occurs in a similar manner to animal cells, but with an additional factor – the cell wall. When a plant cell is placed in a hypotonic solution:
- The influx of water increases the turgor pressure within the cell.
- This internal pressure pushes against the cell wall, making the cell rigid or turgid, which is beneficial for the plant’s structural integrity.
🌱 Note: The term ‘turgor pressure’ describes the pressure exerted by water inside the cell against the cell wall.
Question 2: What Happens to a Cell in a Hypertonic Solution?

When a cell is exposed to a hypertonic solution:
- Water will move out of the cell due to the higher solute concentration outside.
- This leads to the cell shrinking or crenating, which in animal cells can be detrimental or even lethal, while plant cells will wilt, a process known as plasmolysis.
Question 3: The Effects of Tonicity on Red Blood Cells

Here’s a table to demonstrate how tonicity affects red blood cells:
| Tonicity | Effect on Red Blood Cell |
|---|---|
| Isotonic | Cell remains its normal shape. |
| Hypotonic | Cell swells and can potentially lyse. |
| Hypertonic | Cell shrinks and crenates. |

Practical Applications of Osmosis and Tonicity

Osmosis and tonicity have implications beyond the classroom. Here are some real-world applications:
Medical Applications

- IV Solutions: Hospital fluids are formulated to be isotonic to prevent causing harm to patients’ cells.
- Treatment of Dehydration: Oral rehydration solutions are designed with an appropriate balance of salts and sugars to facilitate water absorption in the small intestine.
Food Industry

- Salting Meats: Salt can draw out moisture from bacteria, preventing their growth and preserving the meat.
- Pickling: The high solute concentration in pickling brine ensures food preservation through osmosis.
🍔 Note: Osmosis plays a role in culinary techniques like curing meats or making jams.
To wrap up, understanding osmosis and tonicity isn't just about acing biology exams; it has practical applications that affect our daily lives from health to food preparation. By grasping these concepts, you not only get a better understanding of cell biology but also open your eyes to the underlying mechanisms that keep our bodily functions and even our food supply stable. Remember, cells are constantly working to maintain equilibrium, adapting to external changes, and this dance of movement and balance is what keeps life thriving.
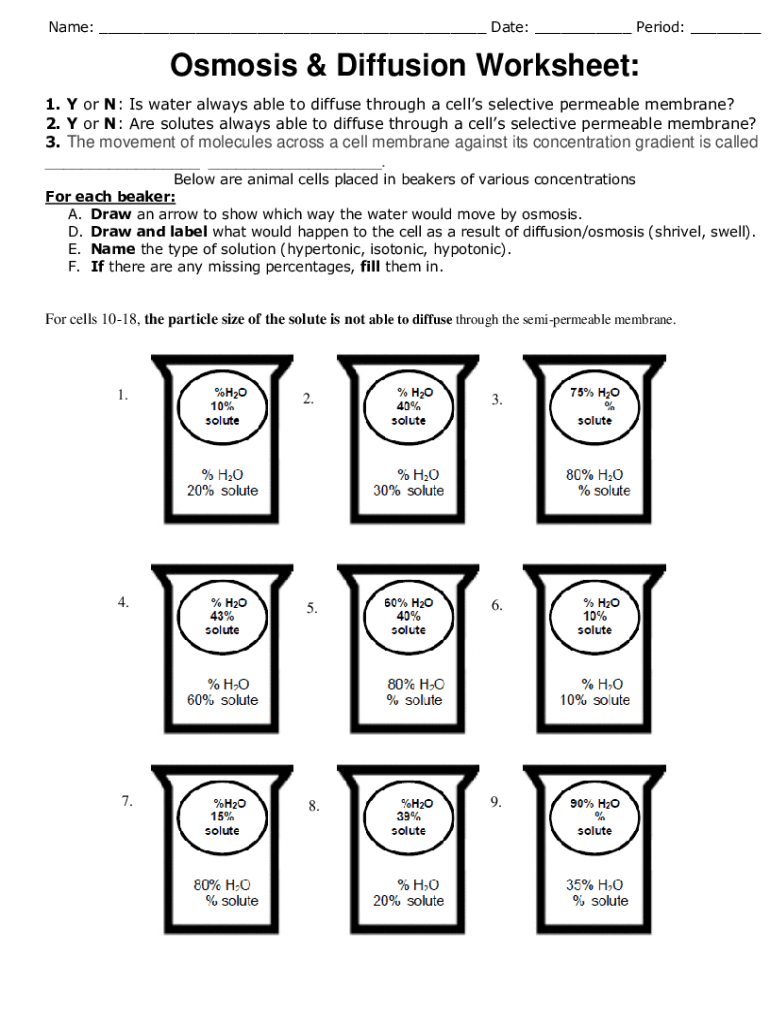
What causes a cell to burst in a hypotonic solution?
+
When a cell is placed in a hypotonic solution, the concentration of solutes outside is lower than inside the cell, leading to water rushing in to equalize concentrations. If this influx of water exceeds the cell’s osmotic capacity, the cell can swell and burst, a process known as cytolysis.
How does osmosis work in dialysis?

+
In dialysis, osmosis is harnessed to remove waste products from the blood. The dialysate, a solution with a controlled concentration of solutes, encourages waste molecules to pass through a semi-permeable membrane from the blood into the dialysate, thus cleaning the blood.
Can you explain the difference between an isotonic and an iso-osmotic solution?

+
An isotonic solution has the same osmotic pressure as inside the cell, so there is no net movement of water. An iso-osmotic solution, on the other hand, has the same concentration of solutes as the inside of the cell, but might not necessarily be isotonic if the solutes have different osmotic potential or if the membrane’s permeability differs for the solutes present.