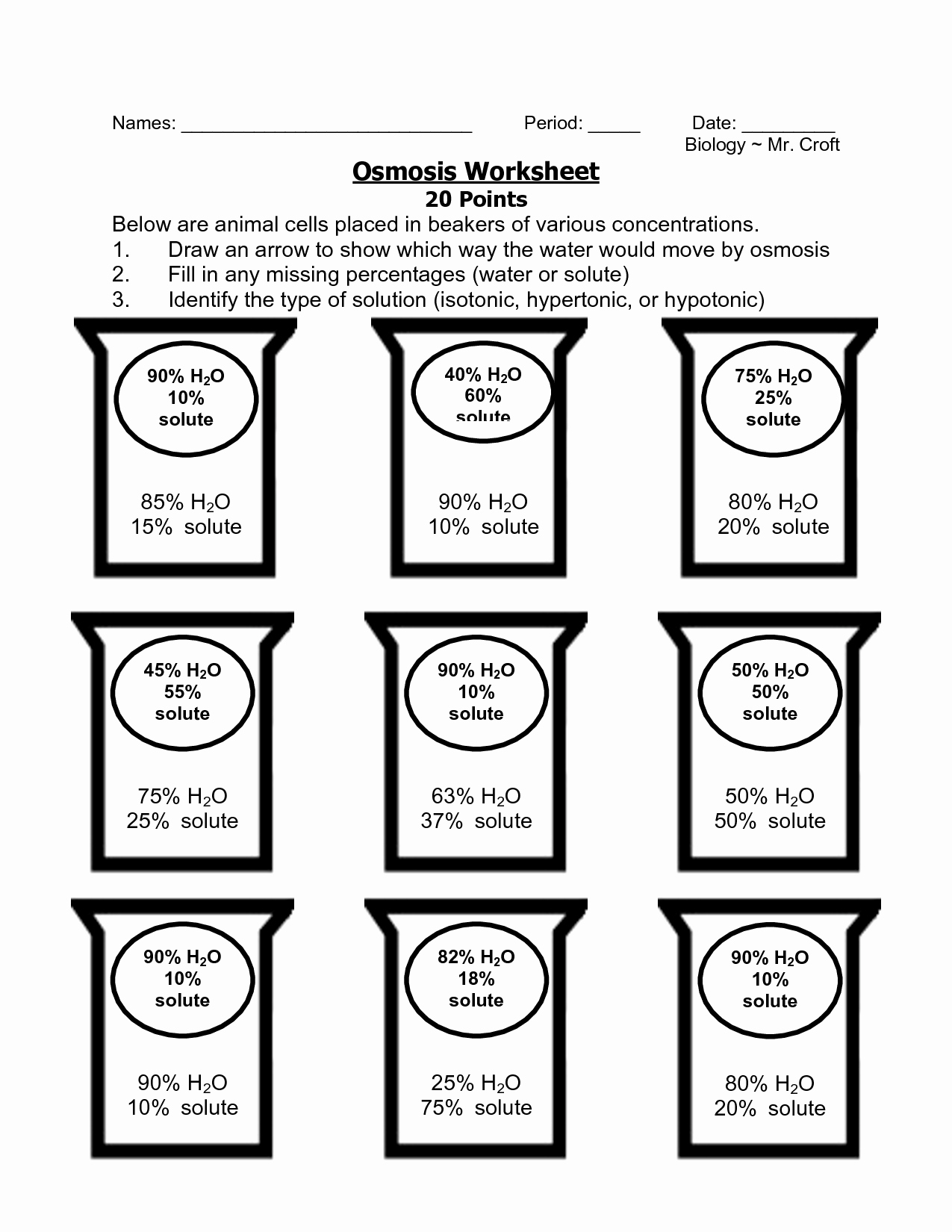
5 Osmosis and Tonicity Worksheet Essentials
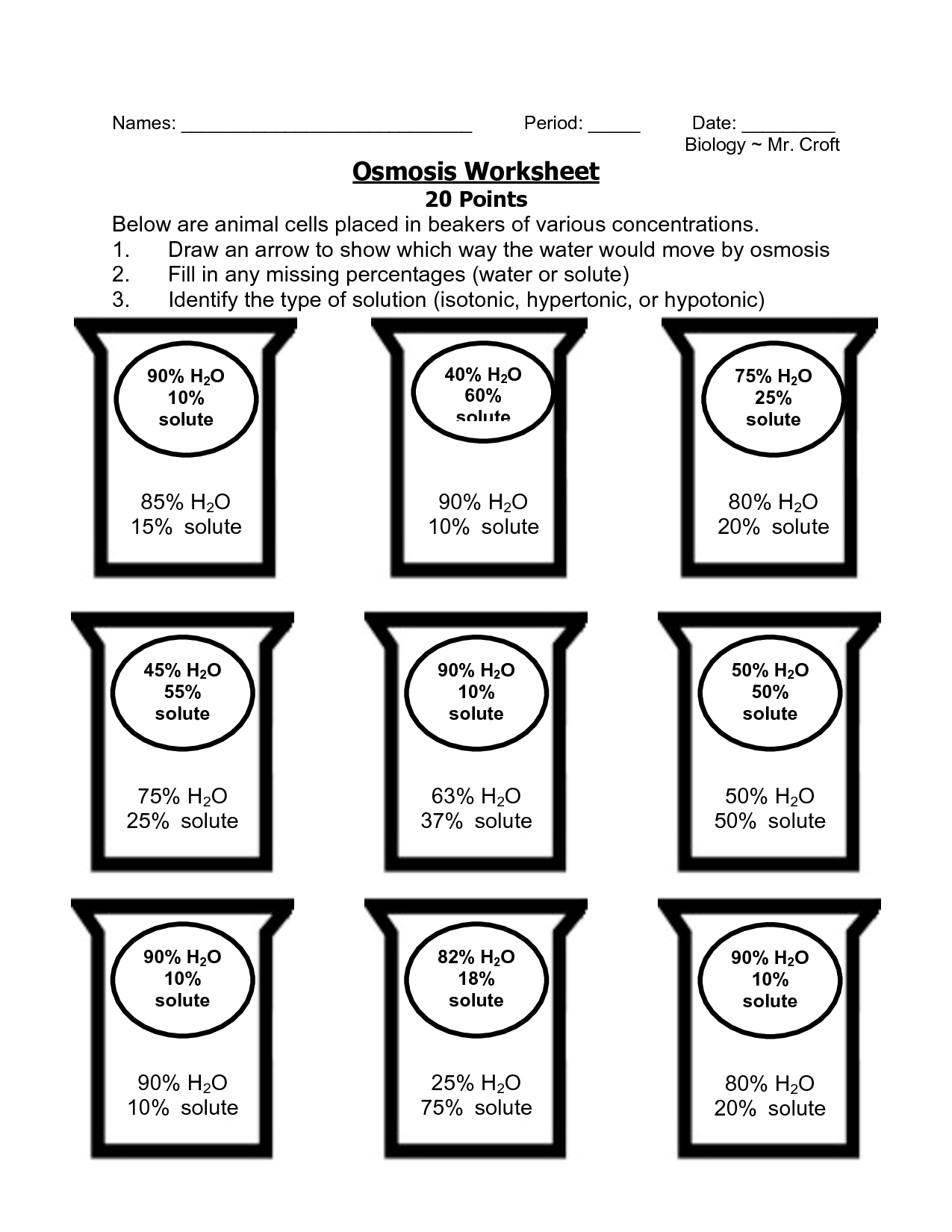
In the realm of biology, understanding osmosis and tonicity is crucial not only for academic purposes but also for practical applications in various scientific fields. These concepts govern how substances move across cellular membranes, influencing cell behavior and functionality. Whether you're a student tackling a biology course or a professional engaged in medical or environmental sciences, mastering these topics can enhance your comprehension of complex biological processes. Here's a detailed exploration into five osmosis and tonicity worksheet essentials designed to deepen your knowledge and ensure accuracy in your scientific explorations.
1. Understanding Osmosis

Osmosis is the passive movement of water through a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. This process aims to balance the solute concentration on both sides of the membrane.
- It’s fundamental in hydration and nutrient absorption in living organisms.
- Plays a role in plant cell turgidity, maintaining cell structure.
- Influences water movement in our kidneys during urine concentration.

💡 Note: Osmosis occurs due to differences in solvent concentration rather than the absolute number of solute particles.
2. Tonicity and Its Types

Tonicity describes the relative concentration of solutes between two solutions separated by a semipermeable membrane:
- Isotonic: Equal solute concentration inside and outside the cell; no net movement of water.
- Hypertonic: Higher solute concentration outside the cell; water moves out, causing cell shrinkage.
- Hypotonic: Lower solute concentration outside the cell; water moves in, potentially leading to cell swelling or even lysis.
| Tonicity | Effect on Cell |
|---|---|
| Isotonic | No change |
| Hypertonic | Shrinkage |
| Hypotonic | Swelling or Lysis |

🧠 Note: Cells often use mechanisms to maintain an optimal internal environment despite changes in external tonicity.
3. Practical Applications

Osmosis and tonicity aren’t just theoretical concepts; they have tangible applications in:
- Medicine: Intravenous solutions must match blood tonicity to prevent cell damage.
- Agriculture: Understanding soil water potential to optimize plant growth.
- Food Preservation: Using osmotic dehydration to preserve food by drawing out water from cells.
4. Experimentation and Observation

Engaging with hands-on experiments is key to understanding osmosis and tonicity:
- Set up experiments with plant cells like Elodea or animal cells to observe changes under different tonicity conditions.
- Use dialysis tubing to simulate semipermeable membranes and track the movement of water or other substances.
5. Problem Solving and Analysis

Worksheets often include problems that require analysis of osmotic scenarios:
- Determine what happens to a cell placed in solutions of varying tonicity.
- Analyze graphs or data sets from experiments to infer osmosis rates and cellular response.
- Calculate osmotic pressure based on given solute concentrations.
The journey through osmosis and tonicity worksheets is enlightening. These worksheets provide a structured approach to learning, offering insights into the mechanics of cellular processes. By mastering these essentials, you gain the ability to predict cell behavior under different conditions, which is indispensable in biology and beyond. Whether you're preparing for an exam or researching physiological responses, the principles of osmosis and tonicity will serve as your foundation. Embrace these concepts, practice with problem-solving exercises, and you'll unlock a deeper understanding of life at its most fundamental level.
What is the difference between osmosis and diffusion?

+
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration, which can apply to any substance. Osmosis is a specific form of diffusion, where water molecules move through a semipermeable membrane towards a region of higher solute concentration.
Why is osmosis important in living organisms?

+
Osmosis is essential for life as it regulates water balance within cells. It helps in nutrient absorption, maintaining turgor pressure in plants, and kidney function in animals for waste removal.
How can I remember the effects of different tonicity?
+
Use the mnemonic SHO (Swell, Hypertonic Out, Optimal): In a hypotonic solution, cells swell; in a hypertonic solution, cells shrink as water moves out; isotonic solutions keep the cell optimal with no net water movement.