Master Orbital Diagrams with This Easy Chem Worksheet

Orbital diagrams are essential tools in chemistry, used to visualize the distribution of electrons within atoms. Whether you're a student grappling with chemistry homework or a chemistry enthusiast wanting to refine your understanding of atomic structure, mastering how to draw and interpret orbital diagrams can significantly enhance your knowledge. In this post, we'll guide you through the process with an easy-to-follow chem worksheet on orbital diagrams.
What is an Orbital Diagram?

At its core, an orbital diagram provides a visual representation of electron configuration in an atom. Here’s how it works:
- Orbits are depicted as circles or squares around the atomic nucleus.
- Each circle or square represents an atomic orbital, which can hold up to two electrons.
- Electron filling follows the Aufbau Principle, Pauli Exclusion Principle, and Hund’s Rule.
Key Principles for Orbital Diagrams
- Aufbau Principle: Electrons occupy the lowest energy orbitals first.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers; hence, each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: When filling orbitals of equal energy (degenerate orbitals), place one electron in each before pairing them up.
Steps to Draw an Orbital Diagram

Drawing an orbital diagram might seem daunting initially, but following these steps can simplify the process:
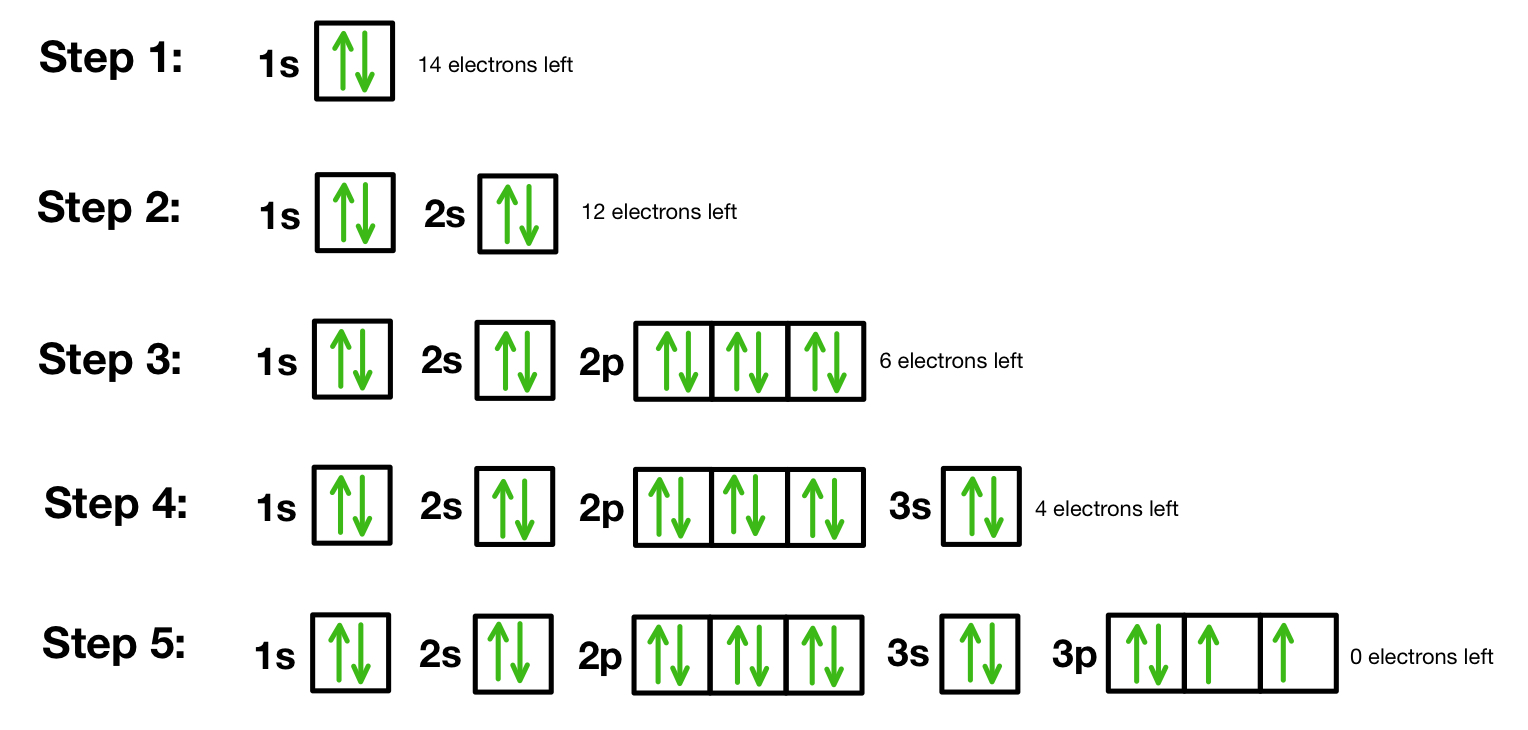
- Determine the number of electrons: The atomic number will tell you this; for example, Nitrogen has 7 electrons.
- Know your shells: The orbitals are organized into energy levels known as shells (1, 2, 3, etc.). Each shell has a different set of subshells: s, p, d, and f.
- Draw the orbital diagram:
- Draw boxes or circles for each orbital in the order of filling: 1s, 2s, 2p, 3s, 3p, etc.
- Label each orbital with its shell and subshell designation.
- Fill the orbitals with electrons:
- Use arrows to represent electrons. The direction of the arrow indicates the electron spin.
- Start filling from the lowest energy orbital.
- Follow Hund’s Rule for equal-energy orbitals.
- Observe the Pauli Exclusion Principle.
- Check and Correct: After filling, review your diagram to ensure it follows all the rules.
An Example: Drawing the Orbital Diagram for Oxygen
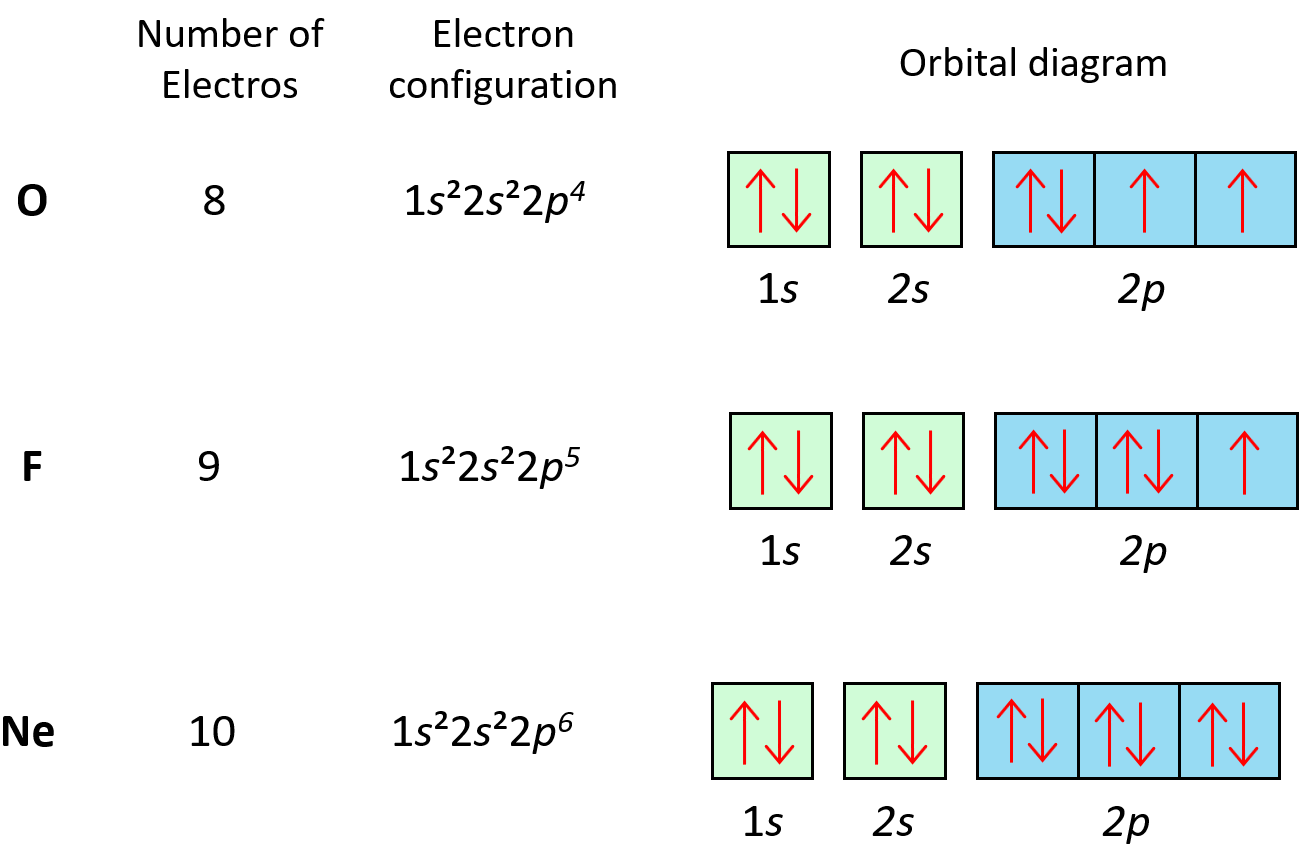
Let’s walk through the orbital diagram for Oxygen:
- Oxygen has 8 electrons, so we start by filling the lowest energy level first.
- We draw the following orbitals: 1s, 2s, 2p (the 2p orbital has three sub-orbitals).
- Place arrows representing electrons into these orbitals:
___ ___ ___ ___ ___ ___
|↑↓| |↑↓| |↑| |↑| |↑|
1s 2s 2p 2p 2p
📝 Note: This diagram illustrates how electrons fill the orbitals according to the rules mentioned. Each arrow represents an electron, and the up or down direction indicates its spin.
Practical Uses of Orbital Diagrams

Orbital diagrams are not just academic exercises; they have practical applications:
- Chemistry Exams: Knowledge of electron configuration is often tested.
- Predicting Chemical Behavior: Understanding electron distribution helps predict how an atom will interact with others in chemical reactions.
- Molecular Orbitals: Orbital diagrams lay the foundation for understanding bonding and anti-bonding molecular orbitals.
As we wrap up our exploration into orbital diagrams, remember that these visualizations are not just about memorizing electron configurations; they are about understanding the behavior and reactivity of atoms. With practice, the process of drawing and interpreting orbital diagrams becomes second nature, providing you with a powerful tool for mastering chemistry. Keep practicing, and soon you'll be able to tackle any element's electron configuration with ease.
Why are orbital diagrams important in chemistry?

+
Orbital diagrams are crucial for understanding how electrons are distributed within an atom, which influences chemical bonding, reactivity, and the properties of elements. They visually represent the quantum mechanical model of the atom.
What does Hund’s Rule state?
+
Hund’s Rule states that within a subshell, electrons will occupy each orbital singly before pairing up with opposite spins. This minimizes electron-electron repulsion and maximizes the total electron spin.
Can you use orbital diagrams to predict chemical reactions?

+
Yes, by understanding the electron configuration, one can predict the stability, chemical reactivity, and potential bonding arrangements of atoms, which are crucial for predicting chemical reactions.