Mastering Orbital Diagrams & Electron Configurations

Understanding atomic structure is fundamental to grasping how atoms interact, bond, and react in chemical reactions. Orbital diagrams and electron configurations are essential tools that chemists use to represent the arrangement of electrons in an atom. This post will explore these concepts comprehensively, offering a step-by-step guide to mastering them, their applications in various fields, and common pitfalls to avoid.
What are Orbital Diagrams?

Orbital diagrams visually depict where electrons reside in an atom or ion. They provide:
- Visualization: Of individual electron shells, subshells, and orbitals.
- Insight: Into electron arrangements which influence an element’s chemical behavior.
An orbital diagram usually uses boxes or lines to represent orbitals and arrows to show the presence and spin of electrons within these orbitals.

💡 Note: For each principal quantum number (n), the number of orbitals can be determined by n2. For instance, the second shell (n=2) has 22 = 4 orbitals.
Electron Configurations: The Basics

Electron configurations describe how electrons are distributed among different atomic orbitals in a shorthand notation:
- 1s2 means the 1s subshell has 2 electrons.
- 3d10 indicates the 3d subshell is completely filled with 10 electrons.
Electron configurations follow three main principles:
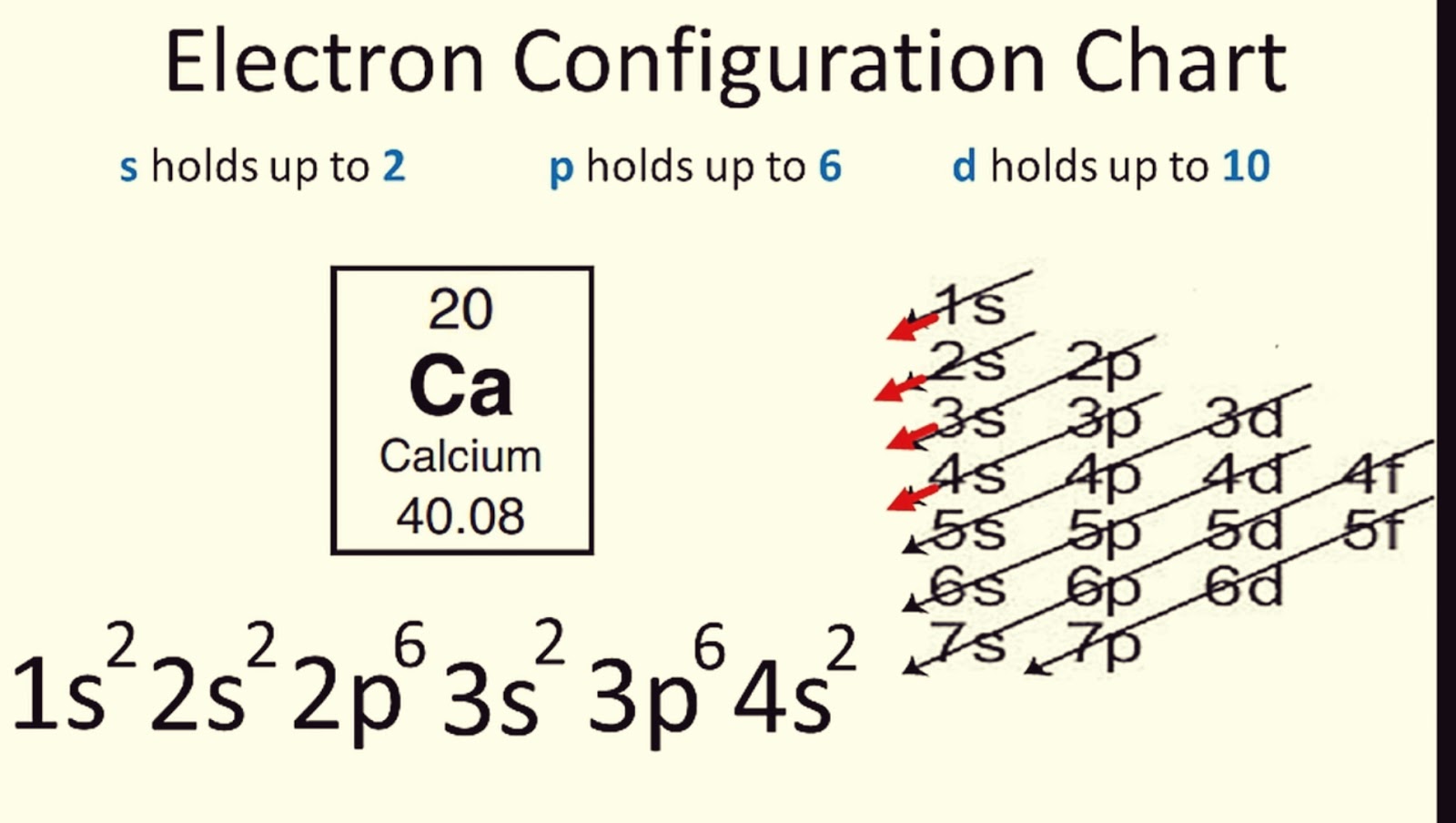
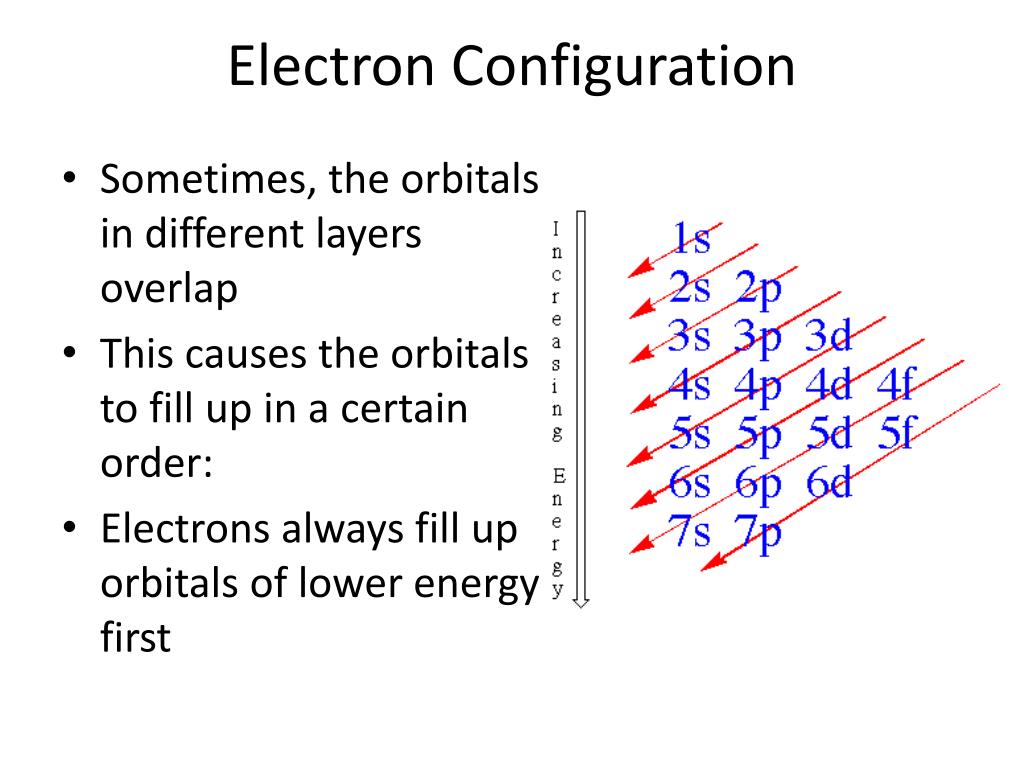
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy levels.
- Pauli Exclusion Principle: No two electrons in an atom can have the same four quantum numbers.
- Hund’s Rule: When filling orbitals of the same energy, electrons will occupy them singly with parallel spins before pairing up.

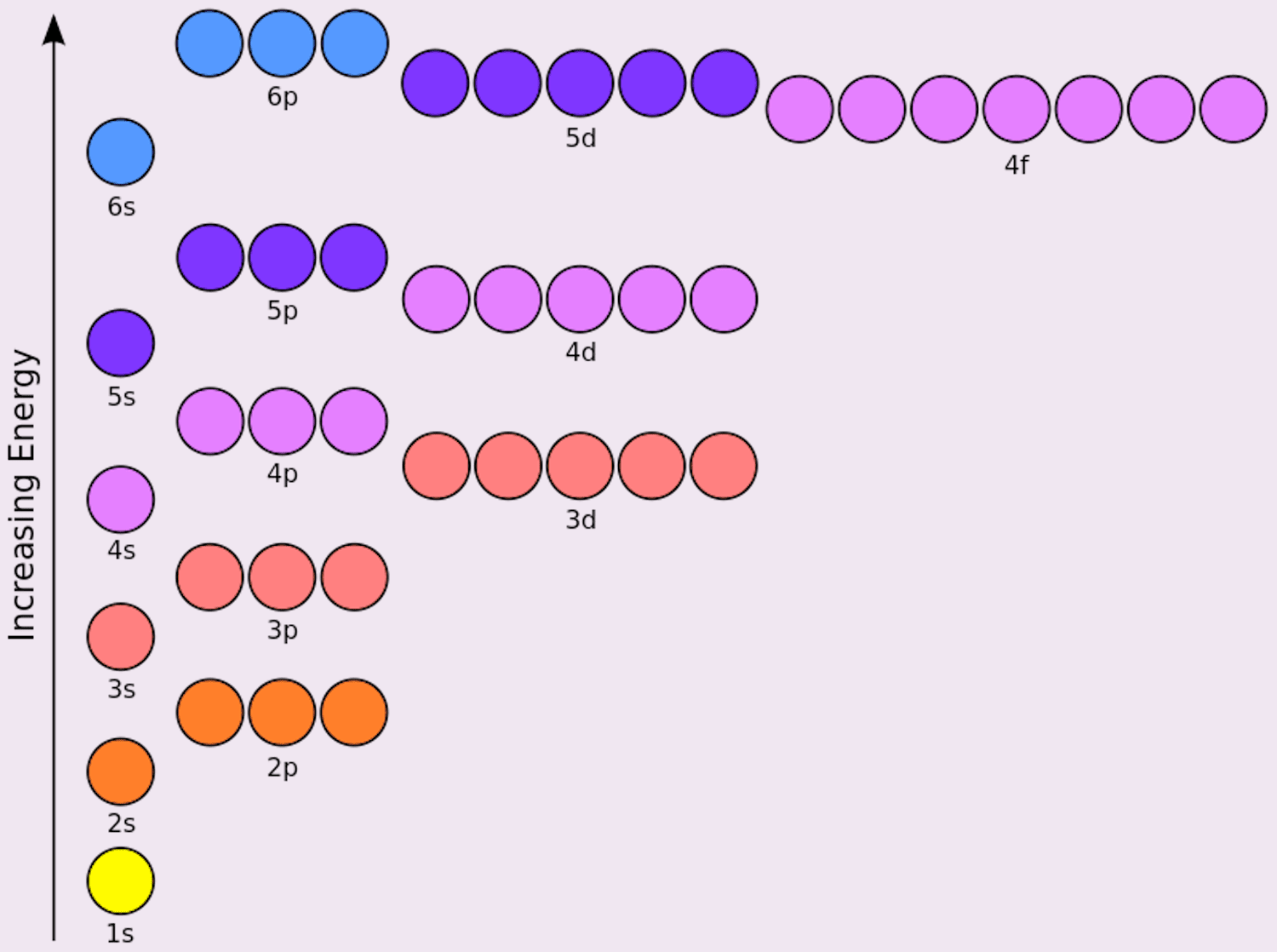
📌 Note: You can remember the Aufbau order using the mnemonic: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d...
Why Do They Matter?
Mastering orbital diagrams and electron configurations is critical for:
- Periodic Trends: Understanding why elements exhibit certain periodic trends like ionization energy or atomic radius.
- Chemical Bonding: Predicting bond types, reactivity, and the nature of chemical bonds based on electron arrangements.
- Spectroscopy: Analyzing atomic spectra which can reveal the electronic structure of atoms.
Constructing Orbital Diagrams & Electron Configurations
Let’s delve into how you can construct both an orbital diagram and an electron configuration for an element:
Step-by-Step Process for Orbital Diagrams

- Determine the number of electrons: Find the atomic number of the element.
- Use Aufbau Principle: Start filling the orbitals in order from the lowest to the highest energy level.
- Apply Pauli Exclusion Principle: Each orbital can hold a maximum of 2 electrons with opposite spins.
- Follow Hund’s Rule: Spread electrons out before pairing them up.
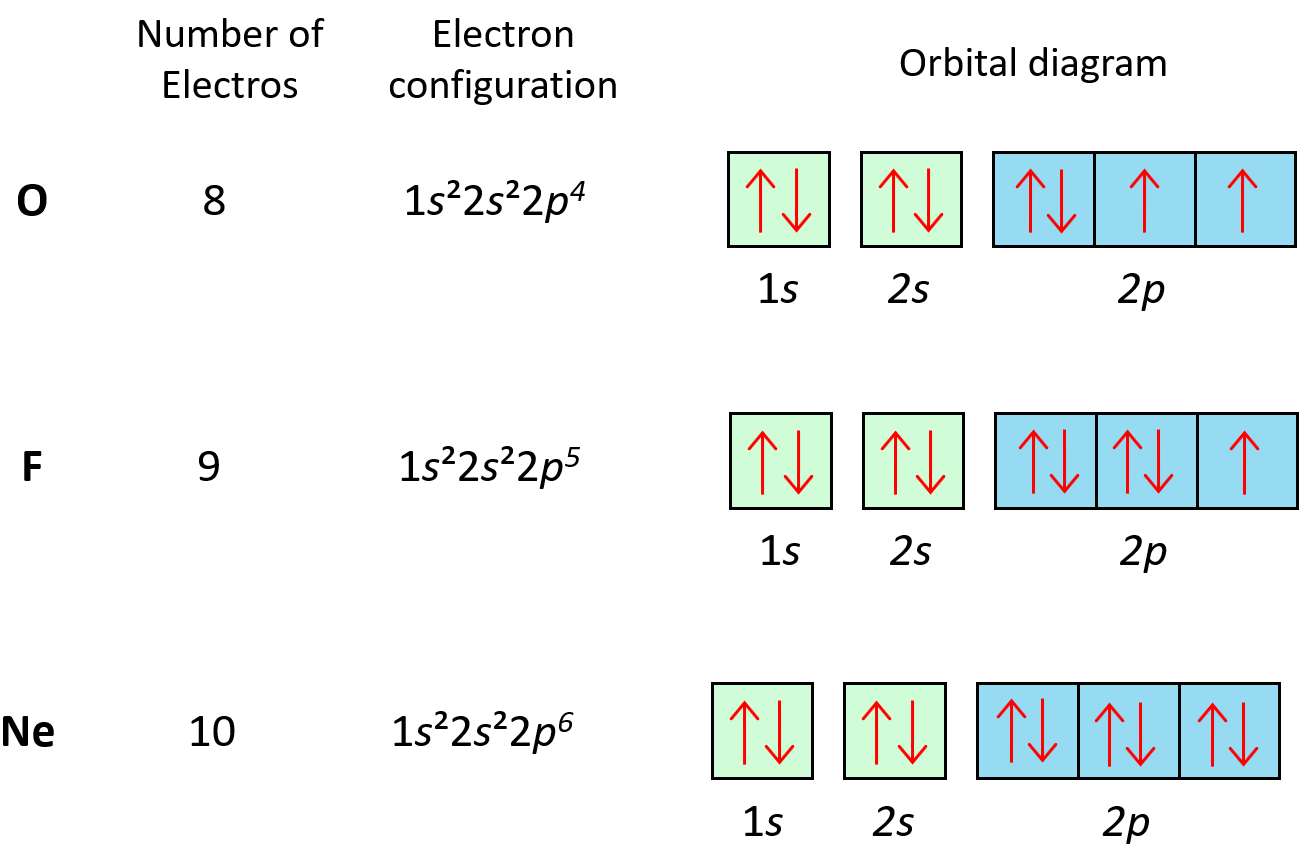
Here’s a simple example for oxygen (O, atomic number 8):
| Shell | Subshell | Orbital Box Diagram |
|---|---|---|
| 1 | 1s | ↑↓ |
| 2 | 2s | ↑↓ |
| 2 | 2p | ↑ |
| ↑ | ||

Electron Configuration for Oxygen

The electron configuration for oxygen using the Aufbau order would be:
1s2 2s2 2p4🔍 Note: Elements in the periodic table are grouped based on their electron configuration, which directly correlates with their chemical behavior.
Advanced Concepts
As we advance, we encounter more complex electron configurations and orbital diagrams:
- Exceptions: Some elements like Cr and Cu don’t follow the simple Aufbau sequence due to the stability of half-filled or fully filled subshells.
- Ions: Electron configurations change when atoms gain or lose electrons to form ions.
- Excited States: When electrons jump to higher energy levels, they exist in excited states, changing the electron configuration temporarily.
Exceptions in Electron Configuration
For instance, Chromium (Cr, atomic number 24) has a slight departure from the expected:
- Expected configuration:
1s2 2s2 2p6 3s2 3p6 3d4 4s2 - Actual configuration:
1s2 2s2 2p6 3s2 3p6 3d5 4s1
🧩 Note: Exceptions occur due to the desire for symmetry in d-orbitals, which offers additional stability.
Electron Configurations in Ions

When atoms form ions, their electron configurations change:
- Cation: Loss of electrons results in a shorter electron configuration.
- Anion: Gain of electrons results in an expanded configuration.
⚡ Note: For multi-electron atoms or ions, electron configurations are often abbreviated using the noble gas notation, e.g., [Ne] 3s2 for magnesium ion, Mg2+.
Practical Applications

Understanding electron configurations and orbital diagrams has practical applications in:
- Material Science: Designing new materials with specific electronic properties.
- Inorganic Chemistry: Predicting coordination complexes and their behavior.
- Environmental Chemistry: Understanding pollutants and their impact at the atomic level.
In this deep dive into orbital diagrams and electron configurations, we've covered the fundamental concepts, explored how they're constructed, and touched upon advanced topics and applications. The elegance of electron arrangements isn't just a theoretical exercise but has tangible implications in the real world, from chemical reactions to material design.
Why are there exceptions to the Aufbau principle?

+
Exceptions occur when there is a small energy difference between electron configurations, leading to a more stable configuration with half-filled or fully filled subshells.
Can an element have more than one electron configuration?
+
Yes, elements can exist in excited states where electrons jump to higher energy levels, giving them multiple possible configurations.
How do electron configurations influence chemical reactivity?

+
The arrangement of electrons determines how easily an atom can lose, gain, or share electrons, directly affecting its reactivity.