Master Nuclear Equations: Worksheet Answers Unveiled
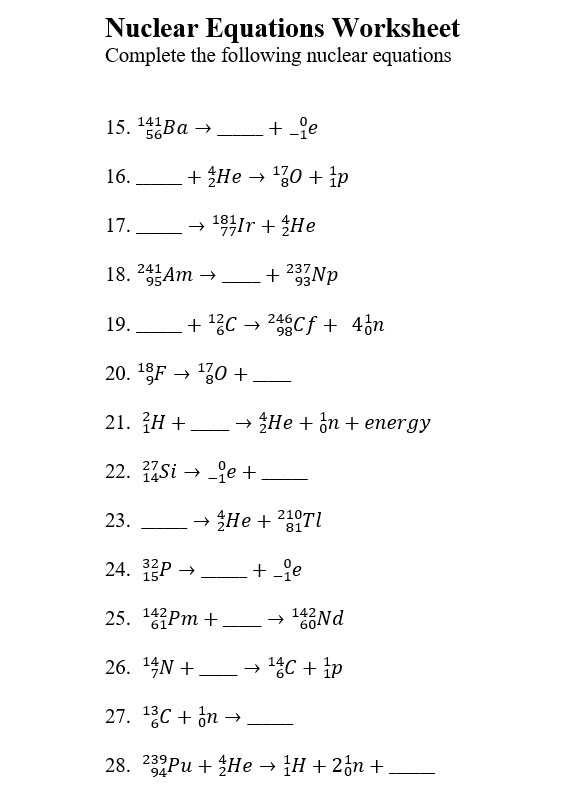
The journey into nuclear chemistry can seem daunting with its complex symbols and calculations. However, by mastering nuclear equations, you not only gain insight into the fundamental processes that power stars and drive technological advances, but also open doors to advanced studies in physics and chemistry. In this extensive guide, we will explore the intricacies of nuclear equations, unveiling answers to common worksheet questions that students often encounter. Whether you are preparing for an exam or simply curious about nuclear reactions, this comprehensive resource aims to clarify and expand your understanding of this fascinating field.
Understanding Nuclear Reactions

Nuclear reactions involve changes in the structure of an atomic nucleus, transforming one nuclide into another. Unlike chemical reactions, which rearrange electrons around the nucleus, nuclear reactions can involve the transformation of protons and neutrons within the nucleus itself. Here are the basic types of nuclear reactions:
- Alpha Decay: When an alpha particle (consisting of two protons and two neutrons) is emitted from the nucleus, decreasing both the atomic number (Z) and the mass number (A).
- Beta Decay:
- Beta-minus (β⁻) Decay: A neutron in the nucleus changes into a proton, emitting an electron.
- Beta-plus (β⁺) Decay: A proton turns into a neutron with the emission of a positron (the anti-particle of an electron).
- Gamma Emission: Often accompanies other decay types, emitting gamma rays which are high-energy photons.
- Fission: The splitting of a heavy nucleus into two lighter nuclei, typically releasing energy and neutrons.
- Fusion: The combining of two light nuclei to form a heavier nucleus, usually with a net release of energy.
🌟 Note: Understanding the conservation laws, especially of mass/energy, momentum, and charge, is fundamental to solving nuclear equations. Remember that the sum of atomic numbers (Z) on both sides of the equation must be equal, as well as the mass numbers (A).
Balancing Nuclear Equations

To balance nuclear equations:
- Identify the reactants and products, noting their mass numbers and atomic numbers.
- Ensure that the sum of mass numbers on the left side equals that on the right, and the same for atomic numbers.
- Use known particles (like alpha, beta, or gamma) to fill in any missing data, maintaining charge and mass balance.
Let’s look at an example:
| Equation | Reactant | Product 1 | Product 2 |
|---|---|---|---|
| ^238U → ^4He + X | ^238U (Z=92, A=238) | ^4He (Z=2, A=4) | X (Z=?, A=?) |

We can solve this by:
- Finding Z for X: 92 = 2 + Z_X (Z_X = 90)
- Finding A for X: 238 = 4 + A_X (A_X = 234)
- Hence, X = ^234Th
Solving Common Worksheet Questions

Here are answers to some typical worksheet questions found in nuclear chemistry:
1. Alpha Decay

Question: Complete the nuclear equation for the alpha decay of ^235U.
Answer:
| Equation | Reactant | Product 1 | Product 2 |
|---|---|---|---|
| ^235U → ^4He + X | ^235U (Z=92, A=235) | ^4He (Z=2, A=4) | X (Z=90, A=231) |
X = ^231Th (thorium-231)
2. Beta-minus Decay

Question: Write the balanced equation for the beta-minus decay of ^14C.
Answer:
| Equation | Reactant | Product 1 | Product 2 |
|---|---|---|---|
| ^14C → X + ^0e− | ^14C (Z=6, A=14) | X (Z=?, A=?) | ^0e⁻ (Z=-1, A=0) |
Here, Z for X would be 6 - 1 + 0 = 5, and A would be 14. Hence, X = ^14N (nitrogen-14)
3. Gamma Emission

Question: A nucleus of ^60Co emits a gamma ray, which does not change its atomic or mass number but releases energy. What is the equation?
Answer: ^60Co → ^60Co + γ
💡 Note: Gamma decay does not change the nuclide's identity, but the energy state of the nucleus is altered.
4. Fission

Question: Complete the following fission reaction where ^235U is bombarded by a neutron:
^235U + n → ^140Xe + … + 3n
Answer:
| Reactant 1 | Reactant 2 | Product 1 | Product 2 | Product 3 |
|---|---|---|---|---|
| ^235U (Z=92, A=235) | n (Z=0, A=1) | ^140Xe (Z=54, A=140) | X (Z=?, A=96) | 3n (Z=0, A=3) |
The total mass number on the right should be 236 (considering one neutron is used in the fission reaction). Hence, X = ^96Sr.
Final Thoughts

In the realm of nuclear chemistry, understanding and balancing nuclear equations not only paves the way for academic success but also provides a glimpse into the energetic processes that define our universe. Through this detailed exploration of alpha decay, beta decay, gamma emission, fission, and fusion, we’ve learned how to approach these problems with logical and mathematical rigor. Remember, the key to mastering nuclear equations lies in understanding the fundamental principles of nuclear physics, where conservation of charge, mass, and energy are paramount. With practice, these equations become less cryptic and more like puzzles waiting to be solved, revealing the intricate dynamics of atomic nuclei.
Why is balancing nuclear equations important?

+
Balancing nuclear equations ensures the conservation of atomic number and mass number, reflecting the principle of conservation of matter and energy in nuclear reactions. It’s crucial for understanding the stability and decay processes of nuclei.
Can you explain the concept of isotopes in the context of nuclear reactions?
+
Isotopes are atoms of the same element with different numbers of neutrons. In nuclear reactions, the specific isotope involved can dictate the type of reaction or decay process due to differences in nuclear stability and energy levels.
What role do alpha and beta particles play in radioactivity?

+
Alpha particles are involved in alpha decay, which is the emission of a helium nucleus to reduce the atomic and mass numbers. Beta particles, on the other hand, can be electrons (beta-minus) or positrons (beta-plus), transforming a neutron into a proton or vice versa, thereby changing the element’s atomic number without significantly affecting its mass number.



