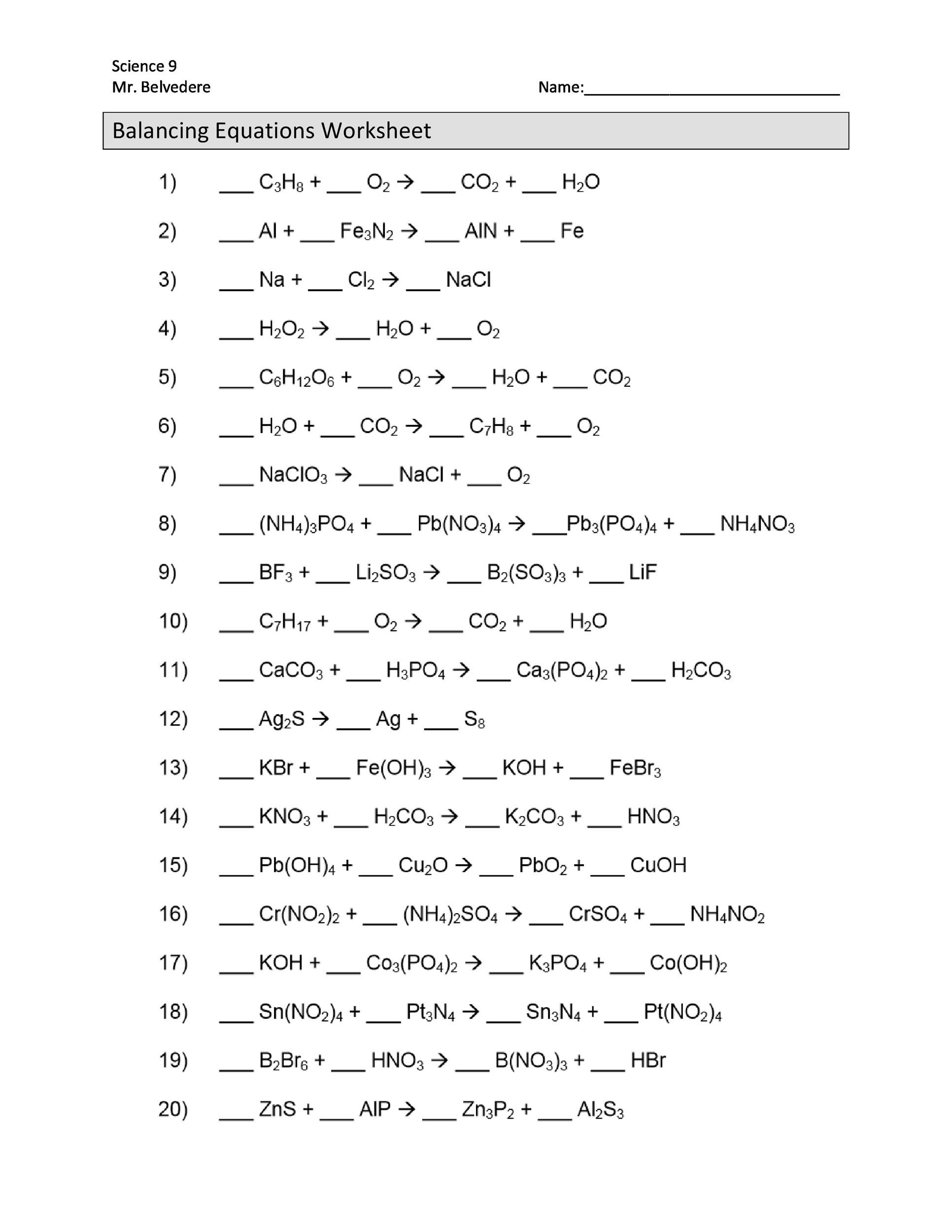
Nuclear Equations Worksheet: Practice Problems and Solutions

Nuclear Equations Worksheet: Introduction
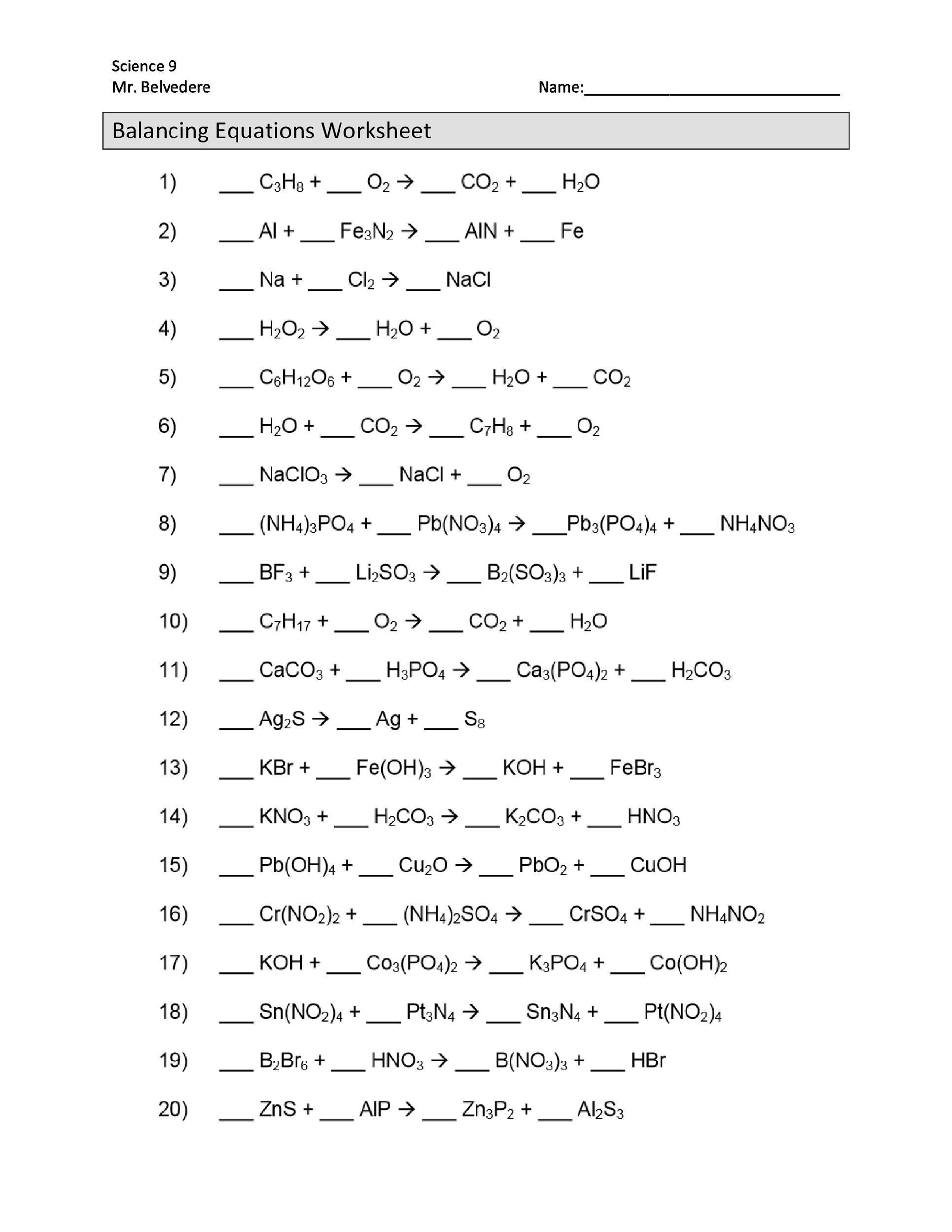
Nuclear equations are a crucial aspect of nuclear physics, representing the changes that occur within an atom’s nucleus during various types of radioactive decay or nuclear reactions. Understanding and balancing nuclear equations is a fundamental skill for students in physics, chemistry, and engineering. This worksheet provides practice problems and solutions to help you master nuclear equations.
What are Nuclear Equations?
Nuclear equations represent the changes that occur in the nucleus of an atom during radioactive decay or nuclear reactions. These equations must be balanced, meaning that the total number of protons (atomic number) and neutrons (mass number) on the left side of the equation must equal the total number on the right side.
Types of Nuclear Reactions

There are several types of nuclear reactions, including:
- Alpha Decay: the emission of an alpha particle (two protons and two neutrons) from the nucleus.
- Beta Decay: the emission of a beta particle (an electron or positron) from the nucleus.
- Gamma Decay: the emission of gamma radiation (high-energy photons) from the nucleus.
- Nuclear Fission: the splitting of a heavy nucleus into two or more lighter nuclei.
- Nuclear Fusion: the combining of two or more light nuclei to form a heavier nucleus.
Practice Problems
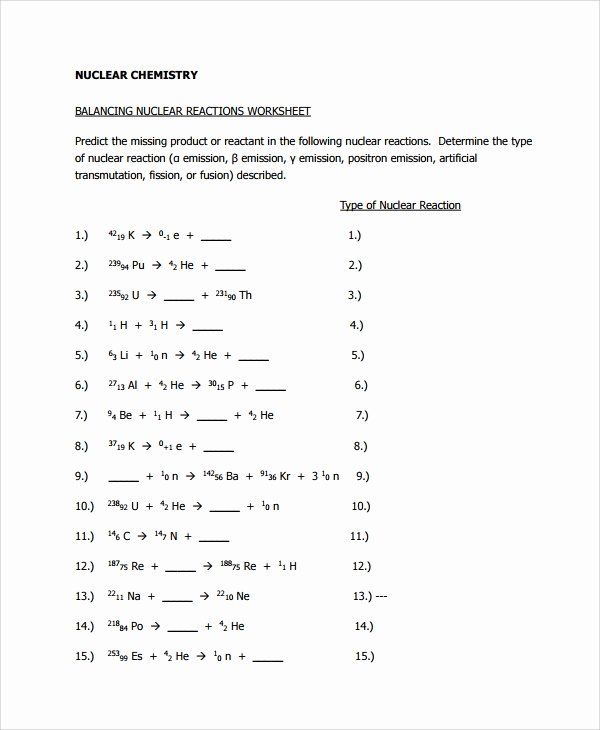
Here are some practice problems to help you balance nuclear equations:
- Alpha Decay: Write the balanced equation for the alpha decay of uranium-238:
238U →?
Solution: 238U → 234Th + 4He
- Beta Decay: Write the balanced equation for the beta decay of carbon-14:
14C →?
Solution: 14C → 14N + 0e
- Gamma Decay: Write the balanced equation for the gamma decay of technetium-99m:
99mTc →?
Solution: 99mTc → 99Tc + γ
- Nuclear Fission: Write the balanced equation for the fission of uranium-235:
235U + 1n →?
Solution: 235U + 1n → 141Ba + 92Kr + 31n
- Nuclear Fusion: Write the balanced equation for the fusion of deuterium and tritium:
2H + 3H →?
Solution: 2H + 3H → 4He + 1n
💡 Note: When balancing nuclear equations, make sure to conserve both the atomic number (protons) and mass number (protons + neutrons).
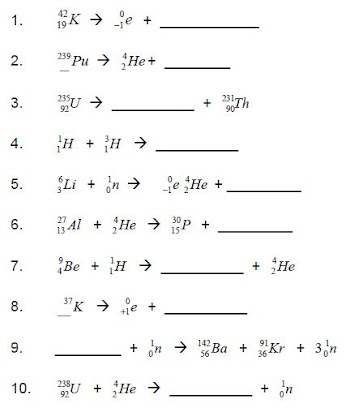
Solutions to Practice Problems
Here are the solutions to the practice problems:
| Problem | Solution |
|---|---|
| 1. Alpha Decay | 238U → 234Th + 4He |
| 2. Beta Decay | 14C → 14N + 0e |
| 3. Gamma Decay | 99mTc → 99Tc + γ |
| 4. Nuclear Fission | 235U + 1n → 141Ba + 92Kr + 31n |
| 5. Nuclear Fusion | 2H + 3H → 4He + 1n |

Conclusion
Balancing nuclear equations requires attention to detail and a deep understanding of nuclear reactions. With practice, you’ll become proficient in balancing nuclear equations and solving problems related to nuclear physics. Remember to conserve both the atomic number and mass number when balancing nuclear equations.
What is the difference between alpha, beta, and gamma decay?

+
Alpha decay is the emission of an alpha particle (two protons and two neutrons) from the nucleus. Beta decay is the emission of a beta particle (an electron or positron) from the nucleus. Gamma decay is the emission of gamma radiation (high-energy photons) from the nucleus.
What is nuclear fission?

+
Nuclear fission is the splitting of a heavy nucleus into two or more lighter nuclei.
What is nuclear fusion?

+
Nuclear fusion is the combining of two or more light nuclei to form a heavier nucleus.
Related Terms:
- Nuclear equations worksheet pdf
- Nuclear equations worksheet answer key
- Balancing nuclear equations worksheet
- Balancing nuclear equations worksheet doc
- Balancing nuclear equations WORKSHEET pdf
- Nuclear decay equations worksheet pdf