Master Nuclear Chemistry with Worksheet K Exercises

Nuclear chemistry, often seen as the pinnacle of scientific complexity, offers fascinating insights into the very atoms that constitute our universe. With its potential applications ranging from medical diagnostics to energy generation, mastering the principles of nuclear chemistry can be both rewarding and daunting. This article delves into Nuclear Chemistry Worksheet K Exercises, providing you with a comprehensive guide to not only understand but excel in this intricate subject.
The Fundamentals of Nuclear Chemistry

Nuclear chemistry studies the behavior of atomic nuclei and the changes they undergo. This includes nuclear reactions such as fission, fusion, and radioactive decay, which can lead to the transformation of one element into another. Here’s a quick overview of the core concepts:
- Radioactive Decay: The process by which an unstable atomic nucleus loses energy by emitting radiation.
- Nuclear Fission: The splitting of a heavy nucleus into two lighter nuclei, often accompanied by the release of a large amount of energy.
- Nuclear Fusion: The combining of two light nuclei to form a heavier nucleus, which also releases significant energy.
Worksheet K: A Deep Dive

Worksheet K typically includes exercises focusing on practical applications and problem-solving in nuclear chemistry. Here’s what you might encounter:
1. Balancing Nuclear Equations

Nuclear reactions, unlike chemical reactions, involve changes in the nucleus, requiring the balance of both charge and mass. Here are a few steps to balance nuclear equations:
- Identify the nuclide before and after the decay.
- Conserve both mass number (A) and atomic number (Z).
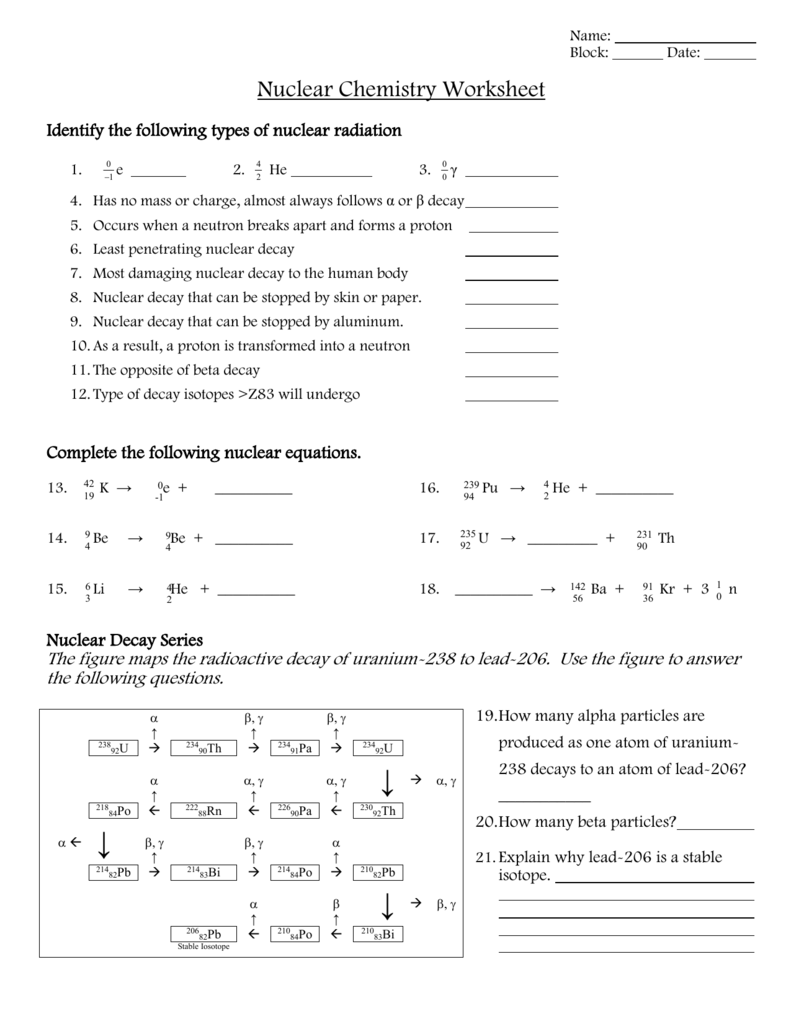
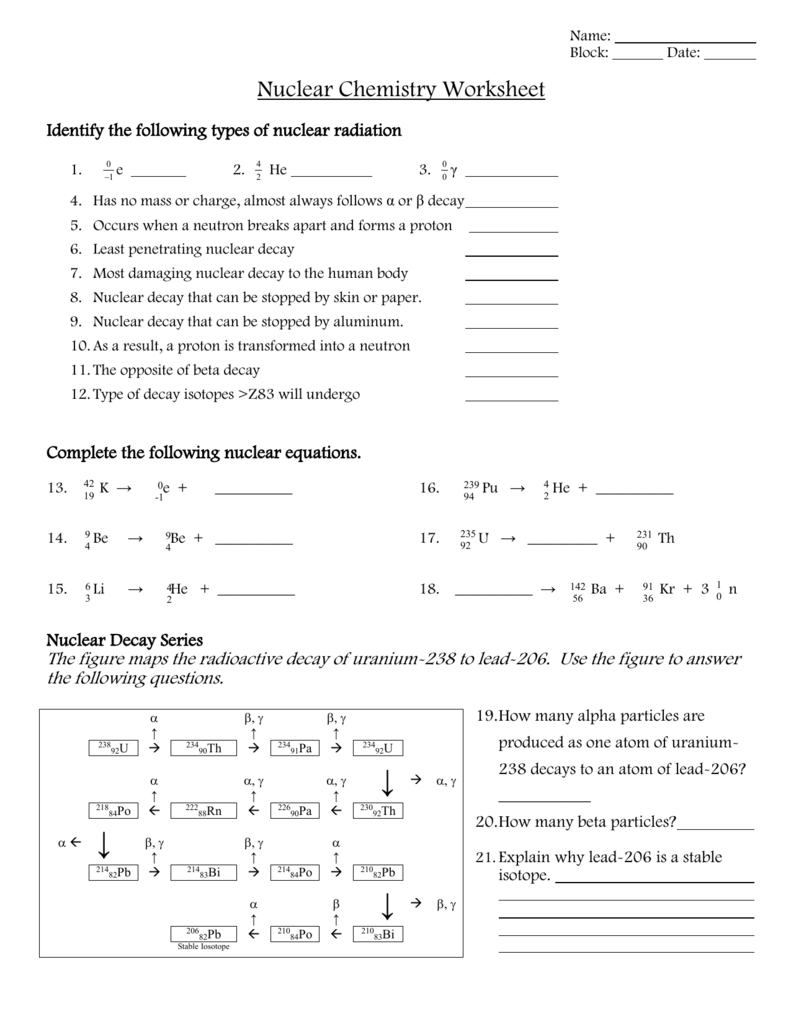
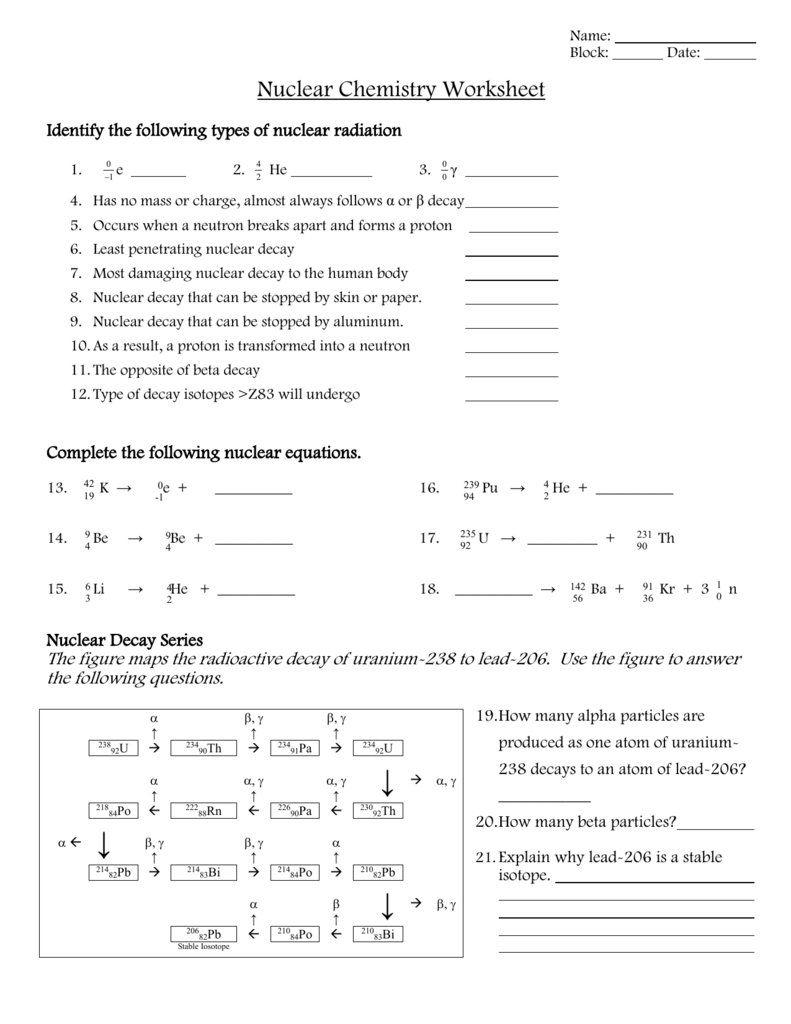
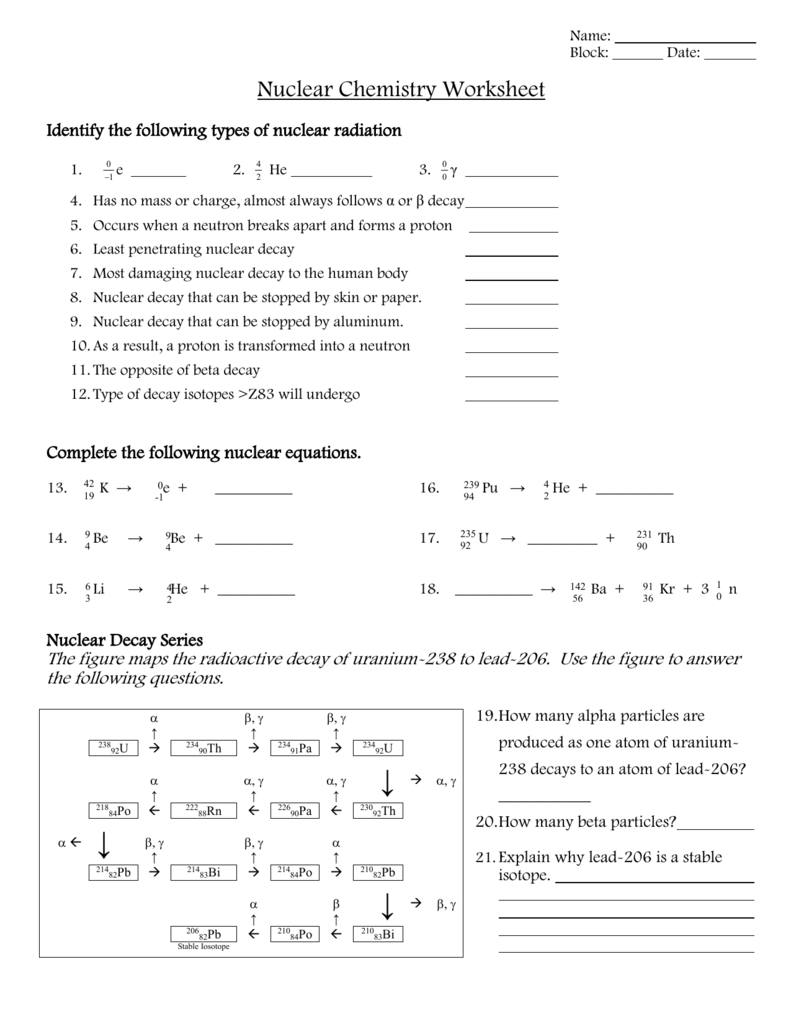
- Adjust for any particles emitted or absorbed during the reaction.
2. Radioactive Half-life Calculations

Understanding and calculating half-life is crucial for predicting the rate of decay. Here are some common questions you might face:
- Determine the half-life from the decay rate.
- Calculate how long it takes for a given amount of substance to decay to a specified level.
- Estimate the number of atoms left after a certain period.
3. Applications of Nuclear Reactions

This section often explores:
- Using radioisotopes in medical treatments like radiotherapy.
- Environmental applications such as dating archaeological artifacts or in soil analysis.
- Energy production in nuclear power plants.
Common Mistakes and Tips
Here are some common pitfalls students encounter in nuclear chemistry:
- Incorrect Balancing: Remember that the sum of the mass numbers and atomic numbers must be the same before and after the reaction.
- Overlooking Isotopes: Pay attention to the isotopes involved since nuclear reactions often involve different isotopes of the same element.
- Miscalculation of Decay Rates: Use the logarithmic nature of exponential decay correctly for half-life problems.
⏱️ Note: Practice with different isotopes and reactions to become comfortable with nuclear equations.
Mastering the Exercises
Here are some strategies to excel in Worksheet K:
- Understand the Theory: Grasp the fundamental concepts of nuclear reactions before attempting exercises.
- Conceptual Mapping: Create flowcharts or concept maps to visualize the relationships between different nuclear reactions and their outcomes.
- Practice with Simulations: Use online tools or software that simulate nuclear reactions to visualize and understand the dynamics better.
| Reaction Type | What to Balance | Example |
|---|---|---|
| Alpha Decay | Mass Number (A) and Atomic Number (Z) |
238U -> 234Th + 4He
|
| Beta Minus Decay | Mass Number (A) and Atomic Number (Z) |
14C -> 14N + 0e- + v
|
| Positron Emission | Mass Number (A) and Atomic Number (Z) |
30S -> 30P + 0e+ + v
|
By consistently practicing with these types of exercises, you'll become adept at recognizing patterns and solving complex nuclear reactions quickly and accurately.
Understanding nuclear chemistry is not just about mastering the calculations and balancing reactions; it's also about appreciating the vast implications of these processes. From powering cities with nuclear reactors to diagnosing diseases, the principles of nuclear chemistry touch upon many aspects of our lives. The worksheet K exercises serve as a practical tool to not only learn but to apply this knowledge in real-world scenarios. They help in sharpening your analytical skills, providing a deeper understanding of how our world functions at its most fundamental levels.
What is radioactive decay?

+
Radioactive decay is a spontaneous process by which an unstable atomic nucleus loses energy by emitting radiation. This emission can result in the transmutation of the atom, transforming it into a new element or isotope.
Why is nuclear chemistry important?

+
Nuclear chemistry is essential for various applications including medical treatments, energy production, and material analysis. It’s also crucial for understanding environmental processes, such as dating archaeological artifacts or tracking the movement of pollutants in ecosystems.
How do you calculate half-life?
+
Half-life can be calculated using the formula: t1⁄2 = (ln(2) * t) / ln(N/N0), where t is the time, N is the current amount of substance, and N0 is the initial amount of substance. This equation allows for estimating the time needed for the remaining substance to decay to half of its amount.