5 Essential Tips for Solving Nuclear Decay Worksheets
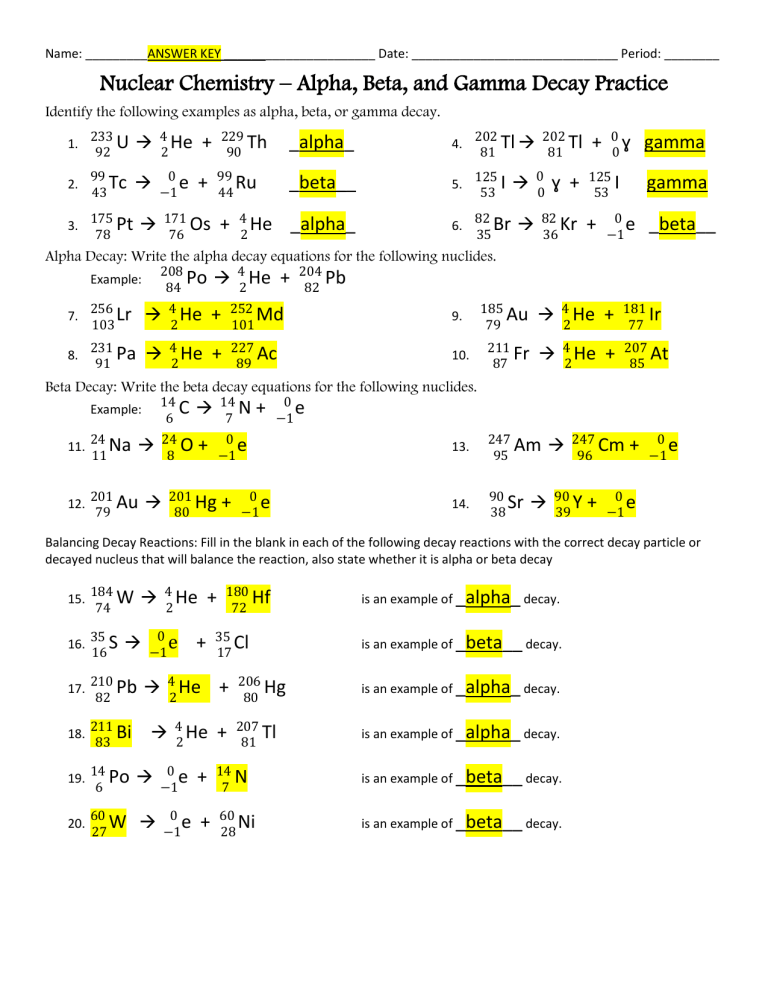
Navigating through nuclear decay worksheets can often seem like deciphering a complex code, especially if you're new to the realm of nuclear chemistry. But fret not! With a clear understanding of the principles and a few essential tips, you can tackle these problems with confidence. Let's explore five tips that will not only help you solve nuclear decay problems but also deepen your understanding of this fascinating area of science.
Understand the Basic Principles

Before diving into any nuclear decay problem, you need a solid grasp of the fundamental concepts:
- Alpha Decay: An alpha particle (α), which is a helium nucleus (2 protons and 2 neutrons), is emitted from the nucleus.
- Beta Decay: Here, a beta particle (β), either an electron or a positron, is emitted when a neutron is converted to a proton (β- decay) or vice versa (β+ decay).
- Gamma Decay: Gamma rays (γ) are emitted when an excited nucleus returns to its ground state, releasing energy without changing the atomic number or mass number.
- Conservation Laws: Key principles include the conservation of mass number (total number of protons and neutrons) and atomic number (number of protons).
Set Up Your Problem Methodically
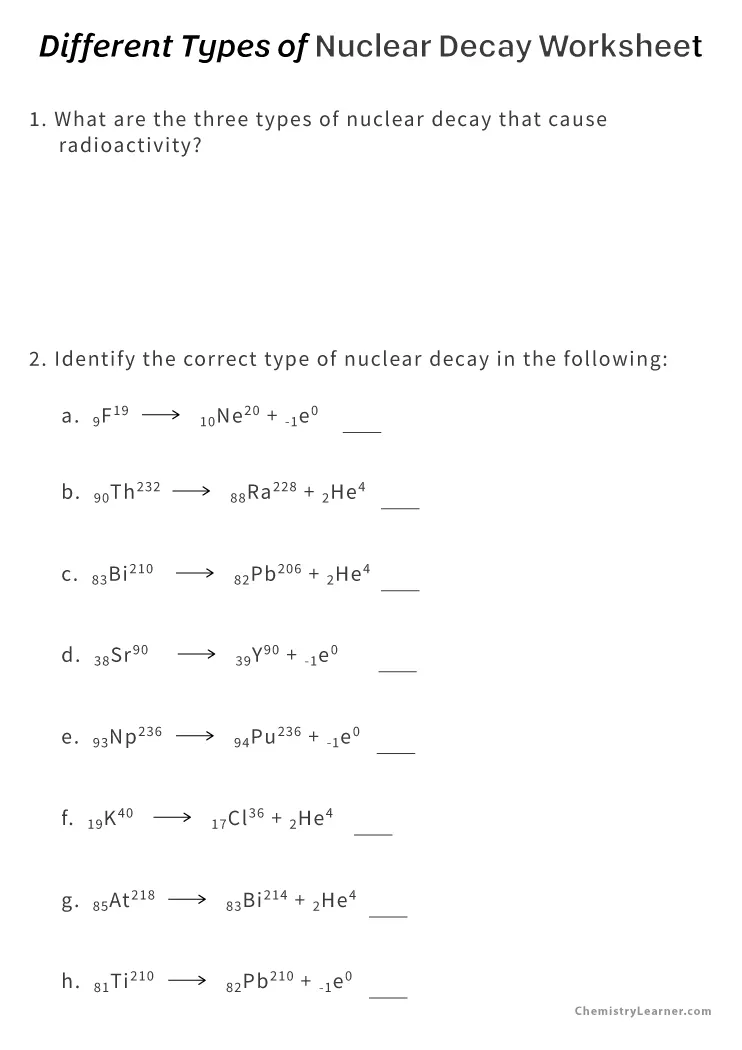
When you encounter a nuclear decay worksheet, follow these steps:
- Identify the type of decay from the question or provided data.
- Write down the isotope involved in the decay.
- Determine what will be the product after decay, considering the changes in mass number and atomic number:
- For alpha decay, subtract 4 from the mass number and 2 from the atomic number.
- For beta-minus (β-) decay, the mass number remains the same, but the atomic number increases by 1.
- For beta-plus (β+) decay, the mass number remains the same, but the atomic number decreases by 1.
- Gamma decay has no change in mass or atomic number but indicates an energy change.
- Balance the equation if necessary, ensuring the sum of the mass numbers and atomic numbers are conserved.
⚠️ Note: For complex decays or when multiple decays occur, track each step individually to keep the calculations accurate.
Use Common Decay Chains
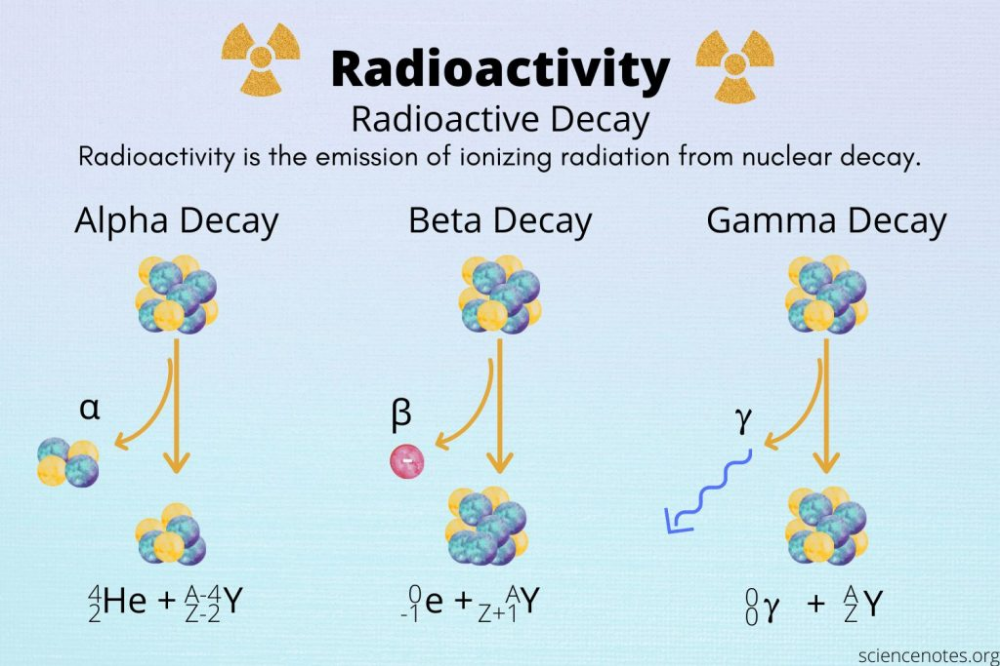
Knowing common decay chains can be very helpful:
| Isotope | Decay Type | Product |
|---|---|---|
| Radium-226 | Alpha | Radon-222 |
| Carbon-14 | Beta-minus | Nitrogen-14 |
| Thorium-234 | Beta-minus | Protactinium-234 |

Practice With Half-Life Calculations

The half-life of a radioactive substance is the time it takes for half of the radioactive nuclei to decay:
- Learn the formula: N(t) = N₀ * (1⁄2)^(t/T₁⁄₂), where N(t) is the amount left after time t, N₀ is the initial amount, and T₁⁄₂ is the half-life.
- Be aware that half-life remains constant for a given isotope, but the rate of decay changes over time.
- Understand how to manipulate this formula for different scenarios, such as when you need to find time or initial amount given other variables.
Verify Your Answers

Before you consider your nuclear decay worksheet complete, go through these verification steps:
- Check that the total mass number and atomic number balance in your equations.
- Ensure the particles emitted and the transformations align with the type of decay indicated in the problem.
- If possible, use known decay chains or series to validate your work.
- If a problem involves half-life, make sure the numbers correlate with the half-life formula.
Once you've become adept at applying these principles and methods, your ability to solve nuclear decay problems will not only improve in accuracy but also in speed. The process of decay and transmutation, once daunting, will become familiar and almost intuitive. Remember that practice is key. As you continue to work through different isotopes and decay processes, you'll find yourself more comfortable with the intricacies of nuclear decay, perhaps even discovering new insights into the subatomic world.
What is nuclear decay?

+
Nuclear decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This often results in the transformation of one element into another.
How does nuclear decay affect the mass of an atom?

+
Nuclear decay can change the mass of an atom. For example, in alpha decay, the mass number decreases by 4 as the nucleus emits an alpha particle. Other types like beta decay do not change the mass number directly but can affect it through subsequent changes in atomic number.
Why is half-life important in radioactive decay?
+
The half-life is crucial because it tells us how quickly a radioactive substance will decay. It’s used to predict how much of the substance will remain after a certain period, which is essential for dating techniques, nuclear physics calculations, and in medical treatments involving radioactive isotopes.