5 Tips for Mastering Monatomic Ions Nomenclature

Understanding the nomenclature of monatomic ions is crucial for students learning chemistry. These ions, which are single atoms with a positive or negative charge, play a fundamental role in various chemical reactions and compositions. In this comprehensive guide, we'll explore five key tips that will help you master the naming conventions of monatomic ions, making chemistry easier to understand and more engaging.
Tip 1: Know the Common Charges

The first step in mastering monatomic ion nomenclature is understanding the common charges of elements. Here’s how you can commit this to memory:
- Halogens: Group 17 elements typically form -1 ions. For example, chlorine becomes chloride (Cl-).
- Alkali Metals: Group 1 elements form +1 ions. Sodium, for instance, forms Na+.
- Alkaline Earth Metals: These are generally +2 ions (e.g., magnesium forms Mg2+).
- Transition Metals: These can have multiple charges, requiring Roman numerals to indicate their state. For example, iron can be Fe2+ (iron(II)) or Fe3+ (iron(III)).
To remember these, you can use mnemonic devices or chemical charts to aid your memorization process.
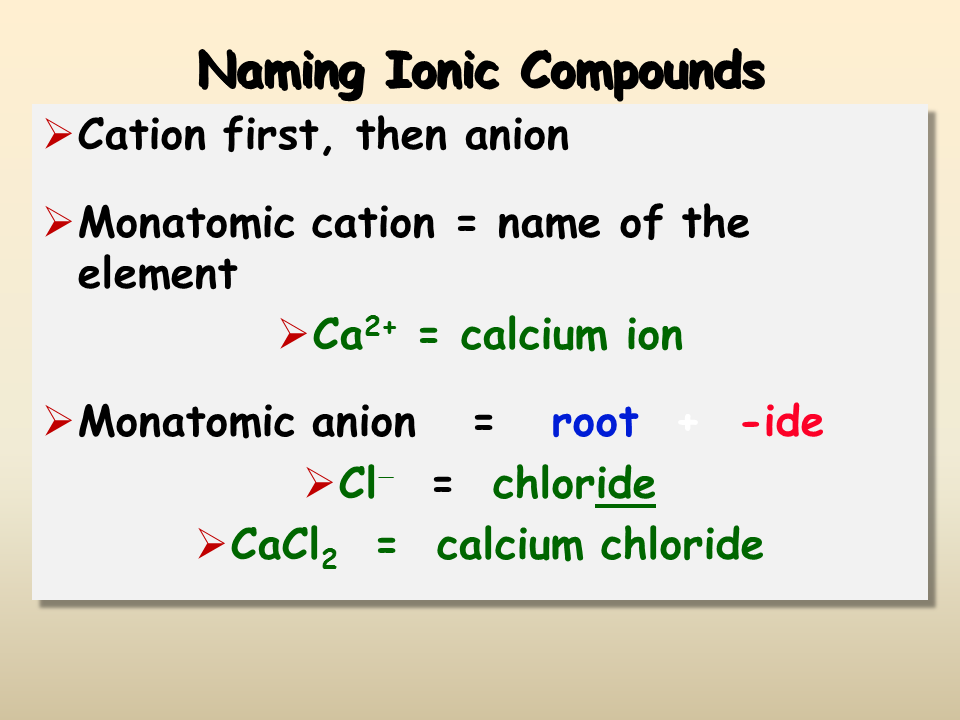
Tip 2: Use Suffixes Correctly

The use of suffixes is critical when naming monatomic ions. Here are the essential points:
- Anions: Add the suffix -ide to the root of the element’s name. For instance, sulfur forms the ion sulfide (S2-).
- Cations: For metals with only one common ion, no suffix is needed. Transition metals with multiple charges use Roman numerals in parentheses to specify the charge.
- Elements from groups 5A through 7A form ions with predictable charges, so understanding group trends will simplify naming.
📚 Note: Be cautious with elements that have multiple potential ion forms. Always check the charge to avoid confusion.
Tip 3: Practice With Examples

Practical application is a key part of learning. Here are some examples for you to practice with:
- Chlorine (Cl) → Chloride (Cl-)
- Magnesium (Mg) → Magnesium ion (Mg2+)
- Iron (III) → Ferric ion (Fe3+)
Utilize online quizzes or workbooks to solidify your understanding. Creating flashcards can also be an excellent tool for memorization.
Tip 4: Understand the Polyatomic Ions

While this tip focuses on monatomic ions, a brief mention of polyatomic ions is necessary:
- Polyatomic ions consist of more than one atom and have specific charges. For example, sulfate (SO42-) or ammonium (NH4+).
- Recognizing the difference between monatomic and polyatomic ions will help prevent confusion when naming compounds.
Remember, polyatomic ions usually retain their name without the usual suffix changes applied to monatomic ions.
Tip 5: Apply Contextual Learning

Monatomic ions don’t exist in isolation; they are part of compounds and reactions. Here’s how contextual learning can enhance your understanding:
- Link the names of ions with their use in everyday life or industrial applications. For example, sodium ions are crucial in body fluids, while chloride ions are common in salt.
- Study chemical reactions involving monatomic ions. Observing their behavior in real scenarios (like oxidation-reduction reactions) will reinforce their names and charges.
Practical examples make learning more engaging and relevant, increasing retention.
In essence, mastering monatomic ion nomenclature involves not only memorization but also understanding the chemistry behind the charges and naming conventions. By utilizing the tips outlined above, you can enhance your proficiency in chemistry, making it easier to tackle more complex topics in the future.
Why is it important to understand the charge of monatomic ions?
+
Understanding the charge of monatomic ions is crucial for predicting how elements will interact in chemical reactions. This knowledge is fundamental in determining the formula and stability of compounds, which is essential for fields like biochemistry, materials science, and environmental science.
How can I remember which metals have multiple charges?

+
A common technique is to use memory aids like mnemonics. For example, remember elements like iron, copper, and lead with a phrase or acronym (like “Iron Copper Lead: 2 or 3?” for Fe, Cu, and Pb). Transition metals often have multiple oxidation states, so reviewing periodic trends can help, too.
Can monatomic ions exist in nature?

+
Monatomic ions are less common in nature due to their high reactivity, but they can be found in ionized states in environments like the atmosphere, interstellar space, or in high-energy conditions. However, they typically combine with other atoms or ions to form compounds which are more stable.