5 Quick Tips for Mastering Ionic Compound Names
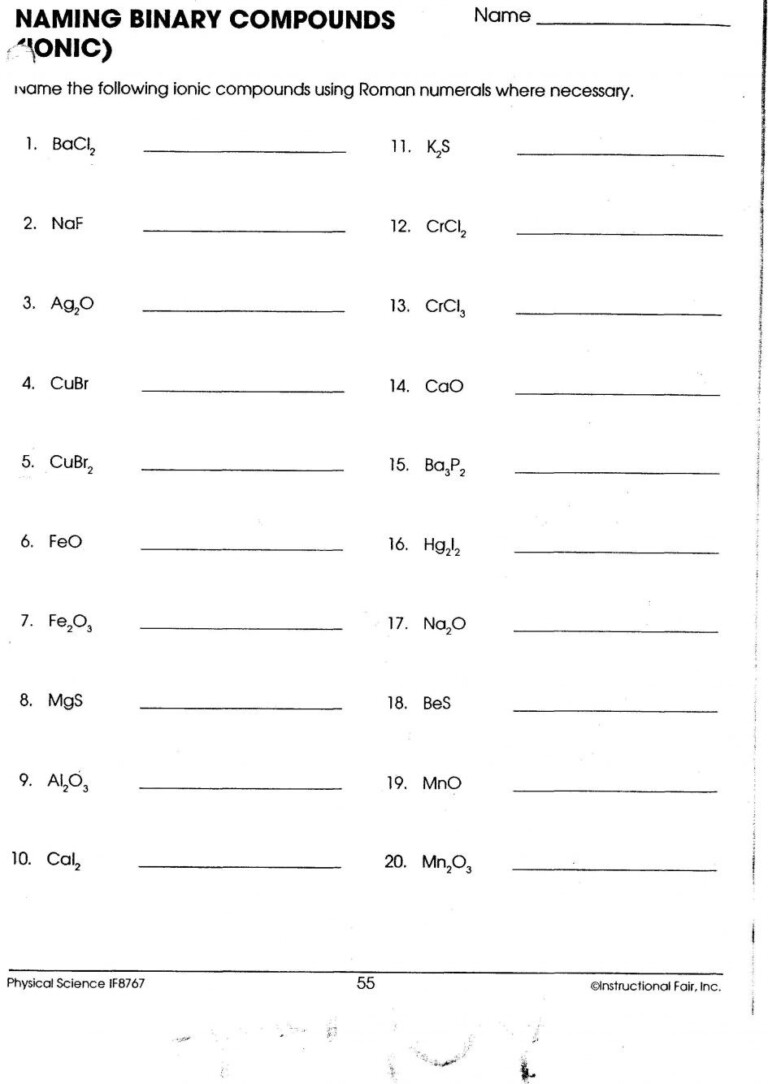
Mastering the art of naming ionic compounds might seem daunting, but with these five quick tips, you'll find yourself effortlessly navigating through the periodic table and beyond. Whether you're a student in chemistry class, a budding scientist, or just someone interested in understanding the science behind everyday products, this guide will serve as your roadmap to ionic compound nomenclature.
Understanding Ionic Bonds

Before diving into names, let’s understand what ionic bonds are:
- Ionic bonds form when one atom transfers electrons to another, leading to the attraction between positively and negatively charged ions.
- Compounds with ionic bonds usually consist of metals and non-metals, where metals lose electrons to become cations and non-metals gain electrons to become anions.

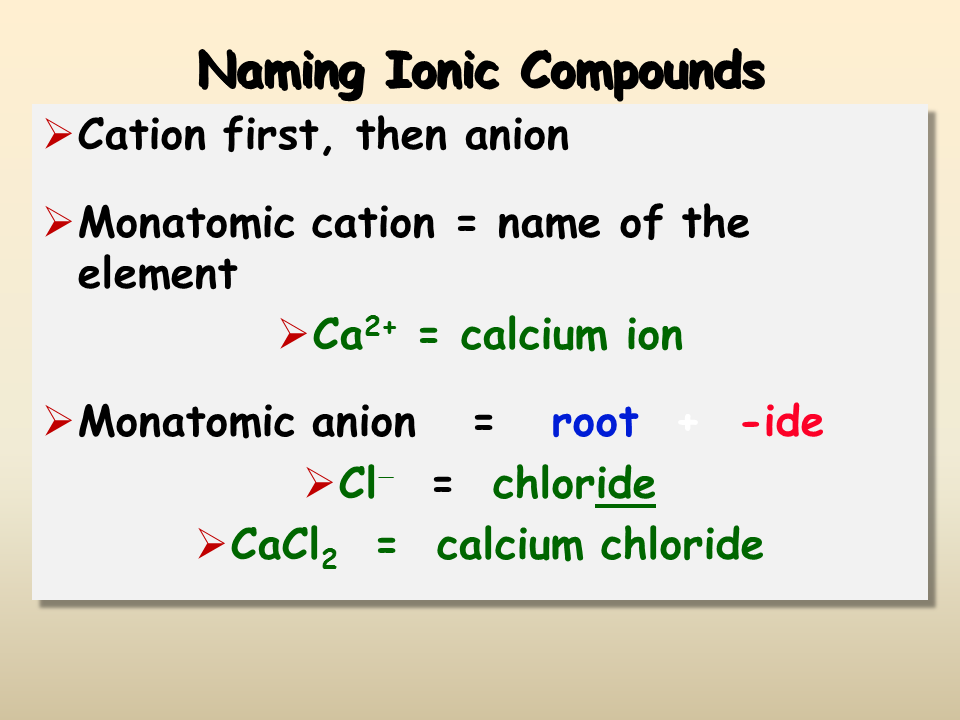
1. Learn the Basics of Naming

The fundamental rule is straightforward:
- The cation (positive ion) is named first.
- The anion (negative ion) follows, typically with the suffix -ide added if it’s a simple monatomic ion. For example, Cl- is chloride.
- Compounds with polyatomic ions have their own specific names, like NO3- for nitrate.
2. Identify Common Ions

Being familiar with common ions will save you a lot of time:
| Ion | Name |
|---|---|
| Na+ | Sodium |
| Ca2+ | Calcium |
| Cl- | Chloride |
| SO42- | Sulfate |
3. Handle Transition Metals with Care

Many transition metals have multiple charges, making their naming a bit tricky:
- Use Roman numerals in parentheses to denote the oxidation state if the metal forms more than one ion. For example, FeCl2 is iron(II) chloride, and FeCl3 is iron(III) chloride.
- Some transition metals use the common ion name system, like copper(II) sulfate for CuSO4.
4. Embrace Polyatomic Ions

Polyatomic ions are complex ions that carry more than one element:
- Recognize common polyatomic ions like carbonate (CO32-), nitrate (NO3-), and phosphate (PO43-).
- If the compound includes a polyatomic ion, the entire ion is treated as a single unit when naming.
5. Practice, Practice, Practice

Lastly, practice is key:
- Regularly work on naming exercises to familiarize yourself with the patterns.
- Use flashcards or online quizzes to test your knowledge on both simple and complex ionic compounds.
Understanding the intricate dance of ions in ionic compounds opens a world of scientific applications, from medicine to environmental science. Whether you're crafting a formula or deciphering a chemical reaction, these steps will guide you towards confidently naming ionic compounds. The more you delve into this fascinating world, the more you'll see the beauty in the complexity of the molecular structures we interact with every day.
Why do we use Roman numerals for transition metals?

+
Transition metals often have multiple oxidation states, and Roman numerals help to specify which charge the metal ion carries in that particular compound, providing clarity and precision in chemical nomenclature.
Can you name an ionic compound with more than two elements?

+
Yes, compounds with polyatomic ions, like calcium carbonate (CaCO3) or potassium nitrate (KNO3), are ionic compounds with more than two elements. The polyatomic ion name remains intact, simplifying the naming process.
What are the steps to name an ionic compound?

+
1. Identify the cation and anion. 2. Name the cation first; if it’s a transition metal with multiple charges, use Roman numerals to indicate its charge. 3. Name the anion, changing its suffix to -ide if it’s a monatomic ion or using the polyatomic ion name.
How do you know which oxidation state to use for metals like iron?

+
The oxidation state of the metal ion can be determined by balancing the charge in the compound. If the metal forms multiple ions, the total charge must equal zero, so the anion’s charge can guide you to the correct oxidation state of the cation.
How can I memorize all these ions?

+
Flashcards, quizzes, and associating ions with everyday examples or mnemonic devices can help. Regular practice with naming exercises also reinforces memory.