Nitrogen Cycle Worksheet: Complete Answers and Insights

Understanding the Nitrogen Cycle

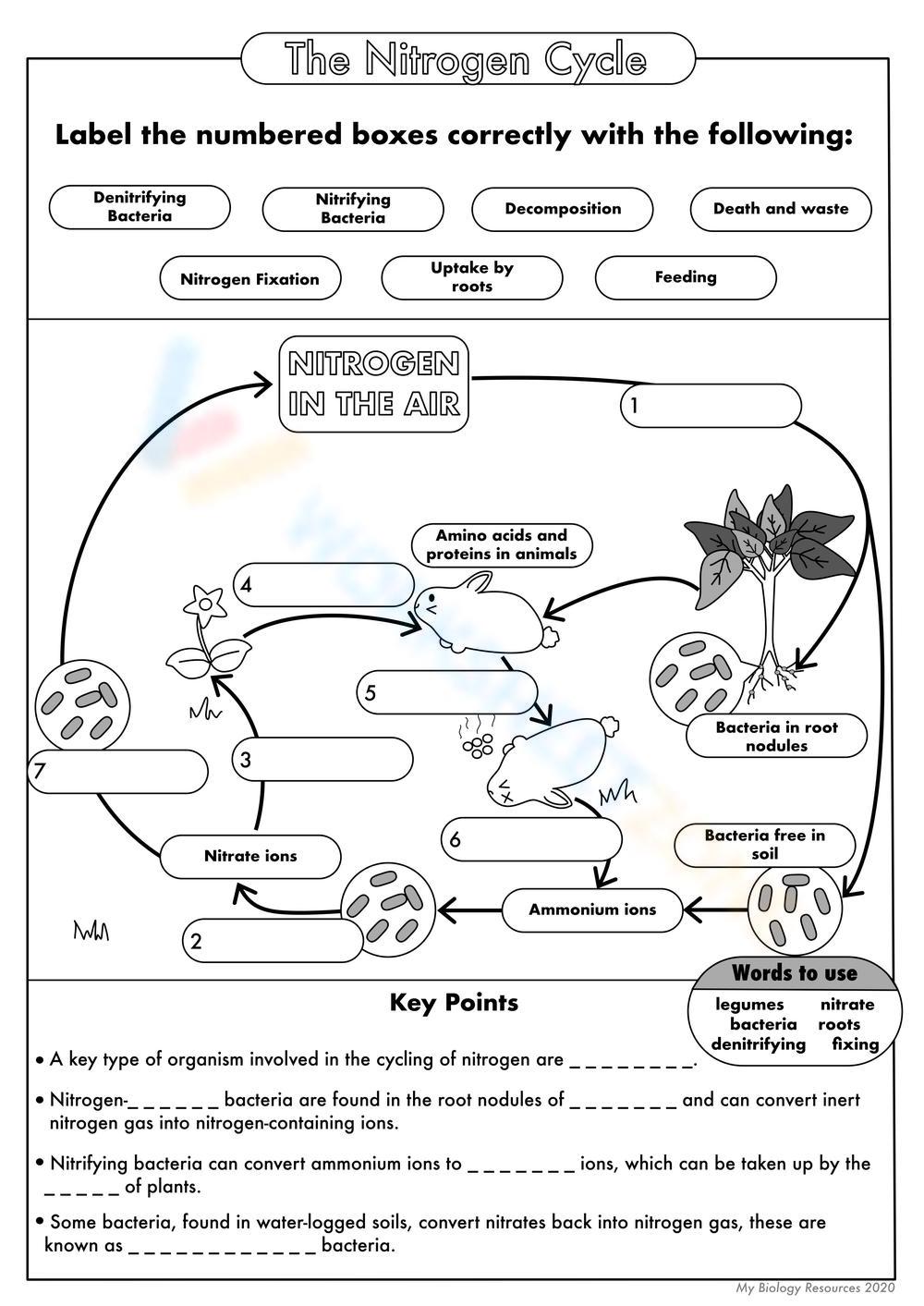
The Nitrogen Cycle is a fundamental biogeochemical cycle that is crucial for life on Earth. Nitrogen, an essential component of amino acids, nucleic acids, and chlorophyll, is required by all living organisms. However, nitrogen exists in several forms in the environment, and its cycling involves complex transformations that are essential for nutrient availability and ecosystem health. Let's dive into the intricacies of the Nitrogen Cycle and understand each step, its ecological significance, and its impact on our planet.

Nitrogen Fixation

The first key step in the nitrogen cycle is nitrogen fixation. Nitrogen gas (N₂) comprises about 78% of the Earth's atmosphere but is not directly usable by most organisms due to the triple bond between nitrogen atoms, which is incredibly stable. Here's how nitrogen fixation occurs:
- Biological Nitrogen Fixation: Certain bacteria like Rhizobium, Azotobacter, and Cyanobacteria convert atmospheric nitrogen into ammonia (NH₃) or nitrates. They do this through enzymes known as nitrogenases.
- Industrial Nitrogen Fixation: Humans contribute to nitrogen fixation through the Haber-Bosch process, which produces ammonia for fertilizers, but this is not part of the natural cycle.
- Lightning-Induced Nitrogen Fixation: During thunderstorms, lightning can provide the energy to break the N₂ bond, leading to the formation of nitrogen oxides which then enter the soil with rainwater.
🌱 Note: Nitrogen fixation by bacteria in root nodules of leguminous plants is an example of mutualism where both plant and bacteria benefit.
Ammonification

Once fixed, nitrogen must be converted into forms accessible to plants. Ammonification is the process by which decomposers break down organic matter, releasing ammonium (NH₄⁺) into the soil. This step involves:
- Decomposition of plant and animal residues.
- Breakdown of urea from animal waste.
- Release of ammonia from industrial and agricultural activities.
Nitrification
Ammonium in the soil is further transformed into nitrites (NO₂⁻) and then into nitrates (NO₃⁻) through nitrification:
- Nitrosomonas bacteria convert ammonium to nitrite.
- Nitrobacter bacteria convert nitrite to nitrate, which plants can easily uptake.
| Stage | Transforming Agent | Nitrogen Compound |
|---|---|---|
| Ammonification | Decomposers | Ammonium (NH₄⁺) |
| Nitrification (First Stage) | Nitrosomonas | Nitrite (NO₂⁻) |
| Nitrification (Second Stage) | Nitrobacter | Nitrate (NO₃⁻) |

⚙️ Note: Nitrification is a two-step process that depends on the presence of both Nitrosomonas and Nitrobacter in the soil.
Assimilation

Nitrates in the soil are taken up by plant roots through assimilation. Plants convert nitrate into amino acids and other nitrogenous compounds, forming proteins, nucleic acids, and other organic materials. This process:
- Allows plants to use nitrogen for growth and reproduction.
- Supports the primary productivity of ecosystems.
Denitrification

On the other hand, denitrification is the process by which nitrates are reduced back into nitrogen gas, effectively removing it from the soil and returning it to the atmosphere. This step is carried out by:
- Bacteria like Pseudomonas and Paracoccus under anaerobic conditions.
- Can lead to a loss of soil fertility if excessive.
Mineralization

In addition to these processes, mineralization involves the conversion of organic nitrogen into inorganic forms like ammonium, making it available for plant uptake again. It happens when:
- Organic matter decomposes.
- Enzymes in soil microbes break down nitrogenous compounds.
⚠️ Note: Excessive fertilizer use can lead to nutrient pollution in water bodies, causing issues like eutrophication.
In sum, the Nitrogen Cycle is a delicate balance of transformation processes that ensure nitrogen is available in ecosystems in forms useful for life. Its comprehension is not only crucial for biology enthusiasts but for all who care about environmental sustainability and agricultural practices.
What is the primary source of nitrogen for plants?

+
Plants primarily obtain nitrogen in the form of nitrates (NO₃⁻) and ammonium (NH₄⁺) from the soil through root uptake.
Why is nitrogen important in ecosystems?

+
Nitrogen is an essential component of amino acids, nucleic acids, and chlorophyll, making it critical for protein synthesis, genetic material, and photosynthesis, respectively, thus driving primary productivity.
Can nitrogen fixation occur without bacteria?

+
While most nitrogen fixation on Earth is biological and relies on bacteria, there are also non-biological ways like lightning and volcanic activity that can fix nitrogen, though they are less significant.