Net Ionic Equation Worksheet Answers: Simplified Solutions for Students

Net ionic equations can be quite challenging for students learning the nuances of chemical reactions. However, they are pivotal for understanding the mechanisms behind reactions in aqueous solutions. Here, we'll dive into a comprehensive guide to net ionic equation worksheet answers, providing simplified solutions that make complex chemistry approachable. Whether you're grappling with dissociation, spectator ions, or the final net ionic form, this blog post is tailored to enhance your learning experience and ensure you grasp the essence of this vital chemistry concept.
Understanding Net Ionic Equations

Before we delve into the answers, let’s clarify what net ionic equations are:
- They represent the true chemical change occurring in a reaction.
- Only species directly involved in the reaction (those not remaining in solution unchanged) are included.
- They show the spectator ions that are not involved in the chemical change.
By removing spectator ions, net ionic equations illustrate the chemistry more concisely, highlighting what changes during the reaction.
Steps to Solve Net Ionic Equations

To generate a net ionic equation, follow these steps:
- Write the balanced molecular equation of the reaction in question.
- Identify all ions that dissociate in an aqueous solution.
- Cancel out spectator ions which appear unchanged on both sides of the equation.
- Write the remaining ions to form the net ionic equation.
🔍 Note: Check the solubility rules to determine which compounds dissociate in water.
Worksheet Examples and Solutions
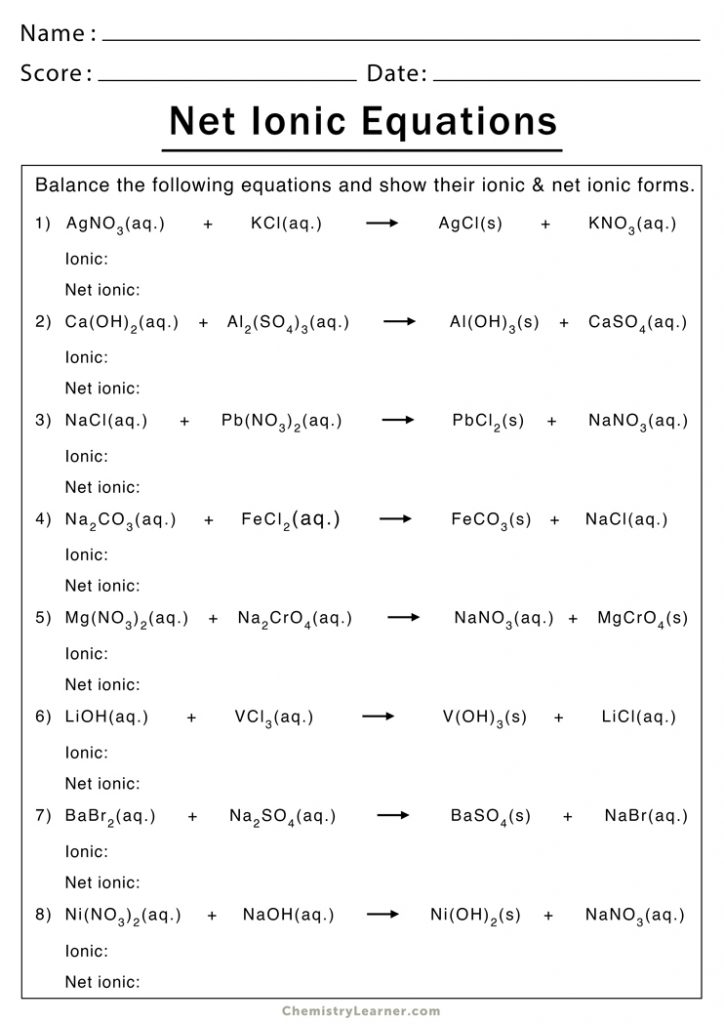
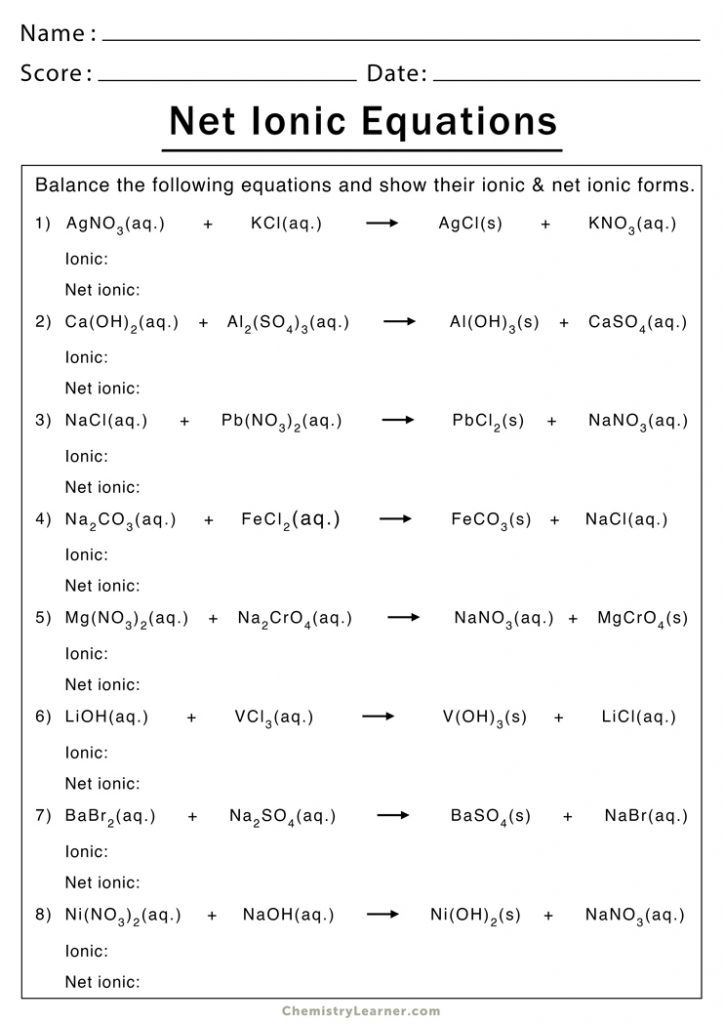
Here are some examples from a typical net ionic equation worksheet, along with their solutions:
Example 1: Precipitation Reaction

Consider the reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl).
- Balanced Molecular Equation: AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
- Ions: Ag⁺(aq) + NO₃⁻(aq) + Na⁺(aq) + Cl⁻(aq) → AgCl(s) + Na⁺(aq) + NO₃⁻(aq)
- Net Ionic Equation: Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
AgCl(s) is the precipitate formed, and Na⁺(aq) and NO₃⁻(aq) are the spectator ions.
Example 2: Acid-Base Reaction

An acid-base reaction occurs between hydrochloric acid (HCl) and sodium hydroxide (NaOH).
- Balanced Molecular Equation: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
- Ions: H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
- Net Ionic Equation: H⁺(aq) + OH⁻(aq) → H₂O(l)
Here, Na⁺(aq) and Cl⁻(aq) are the spectator ions.
Example 3: Gas Evolution Reaction
Hydrochloric acid reacts with calcium carbonate (CaCO₃):
- Balanced Molecular Equation: 2 HCl(aq) + CaCO₃(s) → CaCl₂(aq) + CO₂(g) + H₂O(l)
- Ions: 2 H⁺(aq) + 2 Cl⁻(aq) + CaCO₃(s) → Ca²⁺(aq) + 2 Cl⁻(aq) + CO₂(g) + H₂O(l)
- Net Ionic Equation: 2 H⁺(aq) + CaCO₃(s) → Ca²⁺(aq) + CO₂(g) + H₂O(l)
Common Errors in Net Ionic Equations

To avoid common mistakes, students should:
- Correctly identify if a compound dissociates or not based on solubility rules.
- Ensure all charges balance out in the final net ionic equation.
- Not omit the phase labels (aq, s, l, g) that indicate the state of each ion or compound.
- Check that the equation is balanced in terms of atoms and charges.
💡 Note: Practice writing net ionic equations for various reactions to get a better grasp of the process.
Conclusion

Mastering net ionic equations is a fundamental step in learning chemistry, particularly for reactions in aqueous solutions. By following the steps outlined in this guide and understanding the examples, students can gain a deeper appreciation for the chemical changes that occur at the molecular level. Remember to use solubility rules, keep an eye on spectator ions, and practice regularly. With these tools in hand, you’ll be well on your way to solving any net ionic equation worksheet with confidence and precision.
What is the significance of net ionic equations?

+
Net ionic equations reveal which species are directly involved in a chemical change, thus simplifying complex reactions and focusing on the essential chemical interactions.
Can all reactions be represented by net ionic equations?

+
Not all reactions can be represented by net ionic equations. For example, reactions involving covalent compounds that do not dissociate in water are typically not suitable for this format.
How can I remember which ions dissociate in solution?

+
Utilize solubility rules to memorize common ions and compounds. Most soluble salts, strong acids, and bases will dissociate into ions when dissolved in water.