Master Net Ionic Equations with Our Expert Worksheet
Mastering net ionic equations can seem daunting at first, but with the right approach and tools, it becomes much more manageable. Net ionic equations are a fundamental concept in chemistry, particularly in understanding reactions in aqueous solutions. This article will guide you through the process of solving net ionic equations, providing a comprehensive worksheet to hone your skills. Let's dive into the intricacies of net ionic equations and how you can master them efficiently.
Understanding Net Ionic Equations

Net ionic equations focus on the chemical species that actually participate in a reaction. They simplify the overall equation by omitting spectator ions—ions that appear on both sides of a complete ionic equation, undergoing no net chemical change. Here’s how to approach them:
- Write the Balanced Molecular Equation: Start with the full chemical equation where all the compounds are written in molecular form.
- Convert to Ionic Form: Break down each soluble compound into its constituent ions. Remember, not all compounds dissociate completely.
- Identify Spectator Ions: These are ions that do not change from the reactants side to the products side.
- Write the Net Ionic Equation: Remove the spectator ions, leaving only the essential reactants and products.
Step-by-Step Guide to Solving Net Ionic Equations

Let’s walk through an example to illustrate this process:
Example 1: Sodium Hydroxide + Copper Sulfate

- Balanced Molecular Equation: 2NaOH(aq) + CuSO4(aq) → Na2SO4(aq) + Cu(OH)2(s)
- Convert to Ionic Form:
- 2Na+(aq) + 2OH-(aq) + Cu2+(aq) + SO42-(aq) → 2Na+(aq) + SO42-(aq) + Cu(OH)2(s)
- Identify Spectator Ions: Na+ and SO42- ions.
- Write the Net Ionic Equation:
- Cu2+(aq) + 2OH-(aq) → Cu(OH)2(s)
🎯 Note: This example focuses on precipitation reactions, which is one of several reaction types where net ionic equations are particularly useful.
Example 2: Barium Chloride + Sodium Sulfate

- Balanced Molecular Equation: BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
- Convert to Ionic Form:
- Ba2+(aq) + 2Cl-(aq) + 2Na+(aq) + SO42-(aq) → BaSO4(s) + 2Na+(aq) + 2Cl-(aq)
- Identify Spectator Ions: Na+ and Cl- ions.
- Write the Net Ionic Equation:
- Ba2+(aq) + SO42-(aq) → BaSO4(s)
Common Pitfalls and How to Avoid Them
Here are some common mistakes students make when solving net ionic equations:
- Not Identifying Spectator Ions: Always check both sides of the ionic equation to ensure you’ve excluded all ions that do not change.
- Ignoring Solubility Rules: You must know which compounds dissociate in water. For instance, BaSO4 does not dissociate.
- Charge Balance Errors: Make sure the charges balance in your net ionic equation, as ions carry charges.
- Overlooking Oxidation States: In redox reactions, ensure the oxidation states of atoms change appropriately.
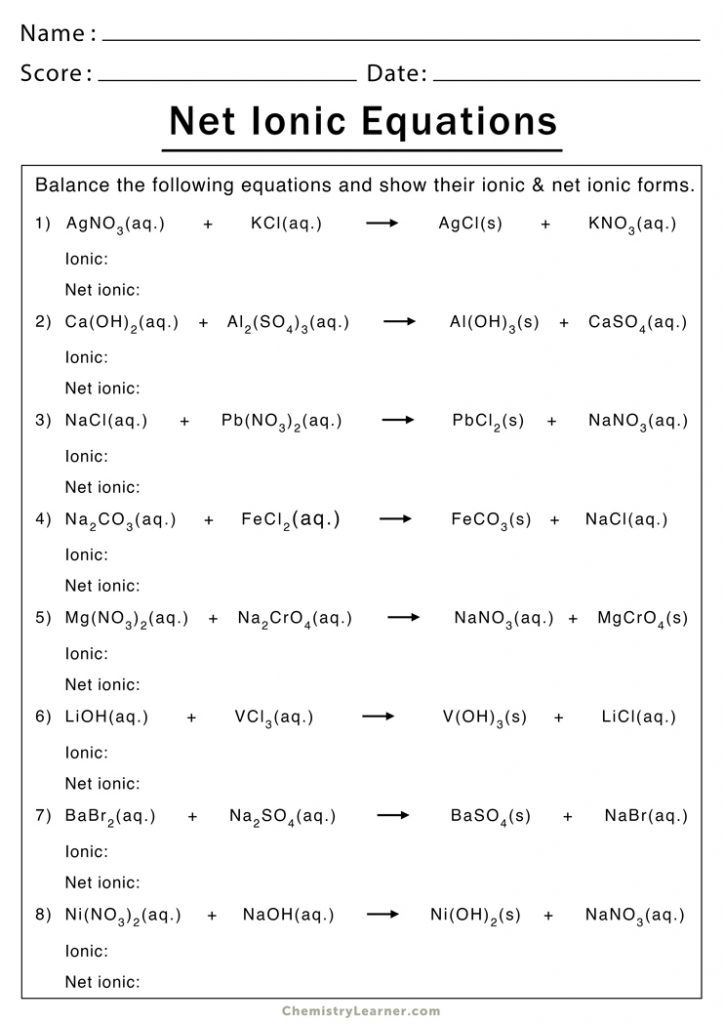
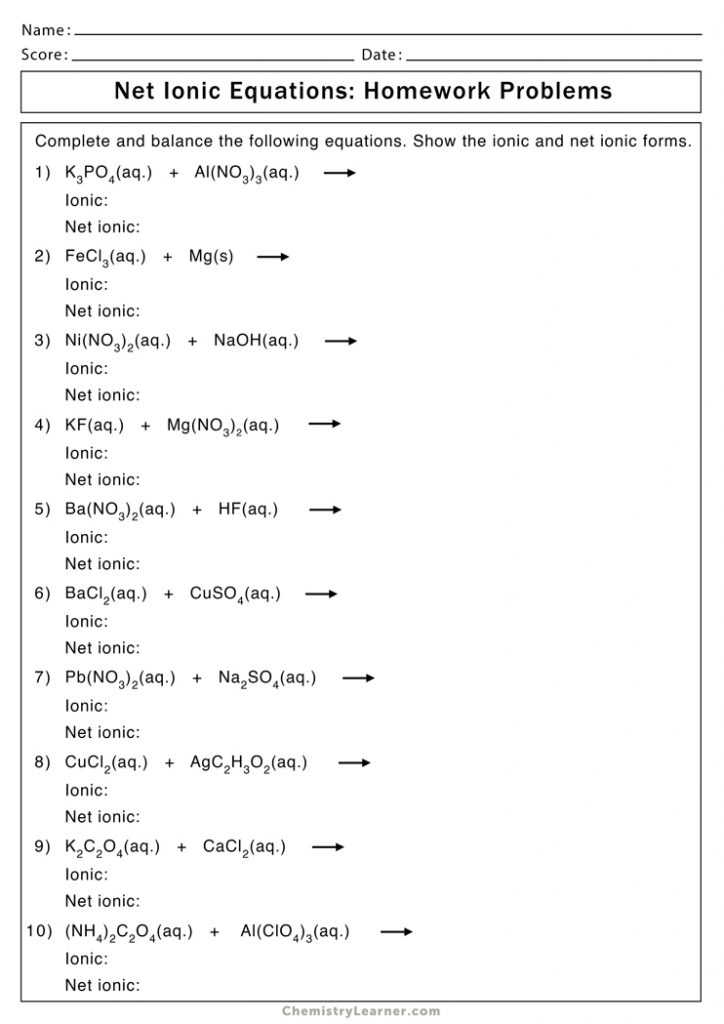
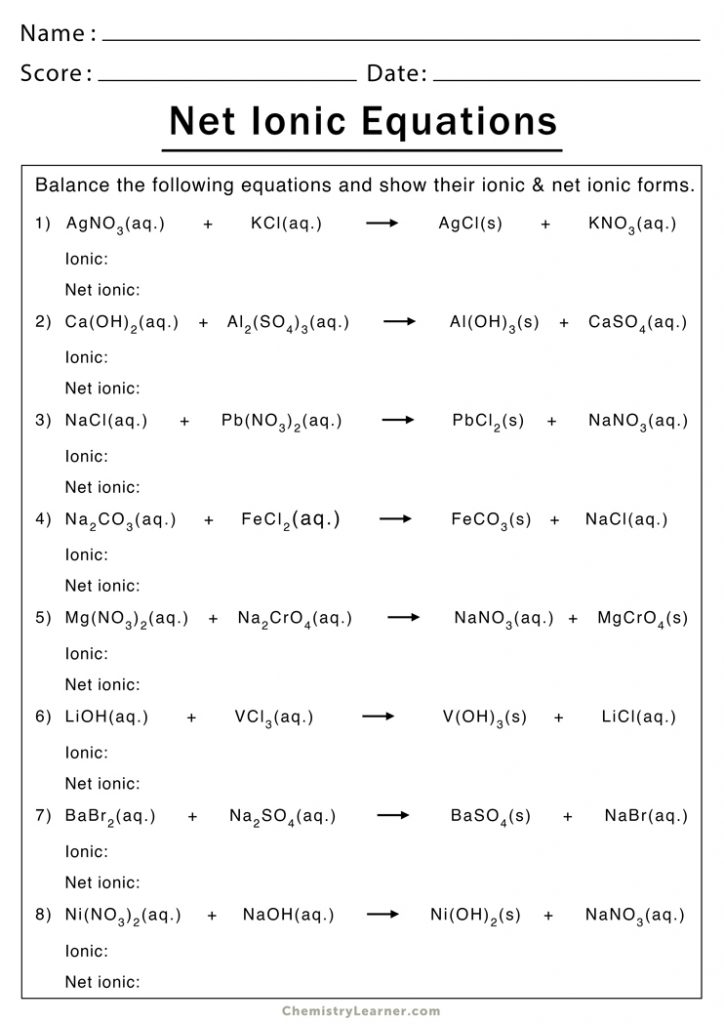
Worksheet for Practicing Net Ionic Equations

Here is a worksheet to help you practice:
| Problem | Balanced Molecular Equation | Net Ionic Equation |
|---|---|---|
| 1 | AgNO3(aq) + KCl(aq) → AgCl(s) + KNO3(aq) | Ag+(aq) + Cl-(aq) → AgCl(s) |
| 2 | Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq) | Pb2+(aq) + 2I-(aq) → PbI2(s) |
| 3 | CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq) | Cu2+(aq) + 2OH-(aq) → Cu(OH)2(s) |

Advanced Techniques for Complex Reactions

Some reactions involve multiple steps or complex ions. Here’s how to handle them:
- Recognize Polyprotic Ions: For acids like H2SO4, consider both ionizations if relevant.
- Understand Equilibrium: Some reactions do not go to completion. Use ICE tables (Initial, Change, Equilibrium) for such cases.
- Redox Balancing: In redox reactions, balance the equation by half-reactions and then identify the net ionic equation.
To round off our discussion, let's reflect on the key aspects of mastering net ionic equations:
- Start with a solid understanding of the molecular equation, focusing on the reactants and products.
- Know the solubility rules to determine dissociation in solution.
- Identify spectator ions accurately by examining which ions remain unchanged throughout the reaction.
- Ensure your net ionic equations are balanced, particularly in terms of charge and atoms.
- Practice with a variety of reaction types to solidify your understanding.
💡 Note: Regular practice with different types of chemical reactions is the key to mastering net ionic equations.
Why are net ionic equations important?

+
Net ionic equations allow chemists to focus on the species directly involved in the reaction, simplifying the understanding of chemical reactions.
How do I identify spectator ions?

+
Spectator ions are those ions that remain in the same form on both sides of a complete ionic equation, undergoing no net change.
Can I use net ionic equations for all types of reactions?
+
Net ionic equations are most commonly used for precipitation, acid-base, and redox reactions in aqueous solutions where ions are involved.