Master the Art of Naming Ionic Compounds Easily

Mastering the art of naming ionic compounds is a fundamental skill in chemistry, essential for understanding the properties and interactions of compounds in various chemical reactions. This skill not only helps in identifying substances in the lab but also underpins much of modern chemical education and practice. In this comprehensive guide, we'll delve into the strategies for naming ionic compounds, providing a detailed walkthrough that can make the process intuitive and straightforward.
Understanding Ionic Compounds

Before diving into the naming conventions, it's crucial to understand what ionic compounds are. Ionic compounds are typically formed when metals react with non-metals, creating substances held together by ionic bonds. These bonds result from the transfer of electrons from the metal (which becomes a cation or positively charged ion) to the non-metal (which becomes an anion or negatively charged ion).
Properties of Ionic Compounds

- Crystalline structure: They often form lattice structures.
- High melting and boiling points: Due to strong ionic bonds.
- Conductivity: When dissolved in water or melted, they conduct electricity.
- Solubility: Many are soluble in water but not in non-polar solvents.
💡 Note: While ionic compounds are generally solids at room temperature, some, like ammonium nitrate, are exceptions.
Naming Ionic Compounds

Simple Ionic Compounds
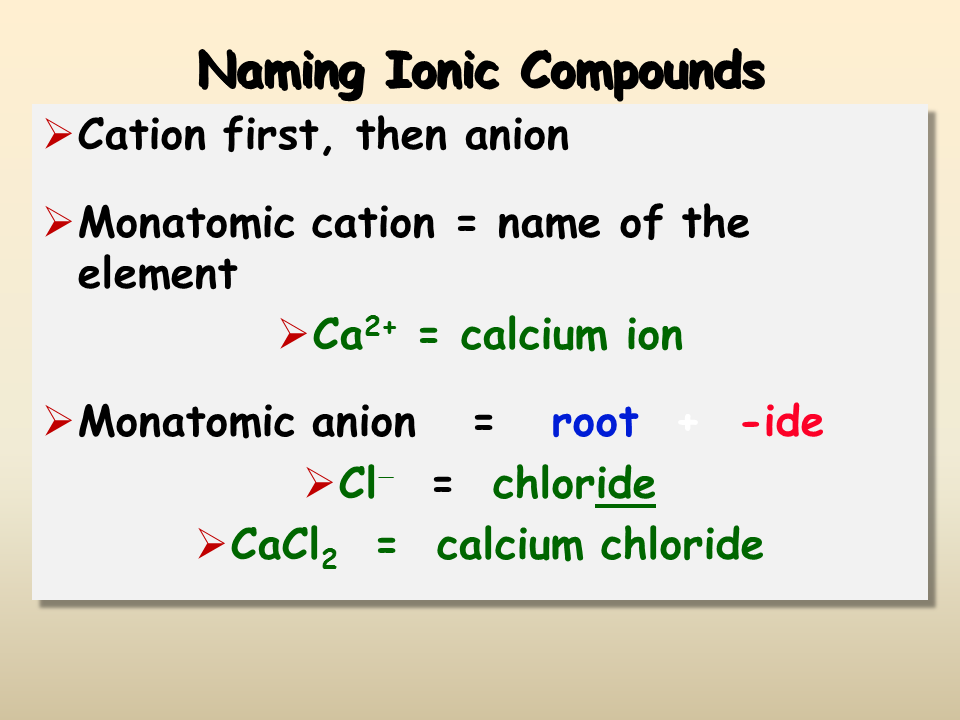
The process of naming straightforward ionic compounds includes:
- Name the metal first: The name of the metal remains unchanged.
- Follow with the non-metal: Replace the ending of the non-metal with -ide. For example, chlorine becomes chloride, sulfur becomes sulfide.
- Keep the stoichiometry in mind: Use prefixes like “di,” “tri” only for covalent compounds unless dealing with polyatomic ions.
Compounds with Transition Metals

When dealing with transition metals, which can exhibit multiple oxidation states:
- Indicate the oxidation state: Use Roman numerals in parentheses after the metal name. For instance, iron in Fe2+ is named iron(II) and in Fe3+ as iron(III).
- Consider common ions: For elements like copper (Cu) or tin (Sn), where the common state might not require Roman numerals, traditional naming systems are also acceptable.
⚠️ Note: The traditional names like cuprous (Cu+) and cupric (Cu2+) are falling out of favor, but you might still encounter them.
Polyatomic Ions

Naming compounds with polyatomic ions follows the same principles, but with a few key considerations:
- Polyatomic Anion: These are groups of atoms that behave as a single unit with a net charge. Examples include nitrate (NO3-) or sulfate (SO42-).
- Keep names intact: Unlike simple ions, the ending of polyatomic ions often does not change. For example, nitrate remains nitrate in compound names.
- Multiple polyatomic ions: If there are multiple units of the same polyatomic ion, you indicate this using prefixes like di-, tri-, etc. For example, Cu(SO4)2 is named copper(II) sulfate with no Roman numeral due to sulfate’s common charge.
| Compound | Naming Rule | Name |
|---|---|---|
| NaCl | Simple Ionic Compound | Sodium Chloride |
| FeCl3 | Transition Metal | Iron(III) Chloride |
| Ca(OH)2 | Polyatomic Ion | Calcium Hydroxide |

Practical Tips for Naming Ionic Compounds

Use Common Ions Cheat Sheet

Having a list of common ions handy can drastically simplify the naming process. Here are some useful points:
- Monoatomic Ions: Understand common charges for elements like sodium (Na+), calcium (Ca2+), and sulfur (S2-).
- Transition Metals: Be aware of typical oxidation states; for example, iron often shows +2 or +3.
Consider Variations

Some ions have multiple common charges or forms:
- Copper: Can exist as Cu+ or Cu2+. Use Roman numerals or traditional names like cupric/cuprous.
- Hydrogen: Forms both H+ (hydride) and H- (proton). The context determines which form is relevant.
In summary, understanding the art of naming ionic compounds involves:
- Recognizing different types of ions: From simple to complex polyatomic structures.
- Applying the correct naming rules: Including the use of Roman numerals for transition metals and understanding the nuances of polyatomic ions.
- Using practical tools: Cheat sheets for common ions can be invaluable.
- Adapting to changes in conventions: Keeping up with modern naming practices while understanding historical naming.
This guide has provided a thorough approach to mastering the naming of ionic compounds, from basic principles to advanced considerations. By mastering these skills, chemists can navigate the complex world of chemical nomenclature with ease, ensuring accurate communication within the scientific community.
What is an ionic compound?

+
An ionic compound is a compound formed from the attraction between two oppositely charged ions, typically a metal cation and a non-metal anion. These compounds are held together by ionic bonds, where electrons are transferred from one atom to another.
Why do some elements use Roman numerals in their names?

+
Roman numerals are used in the names of transition metals to indicate their oxidation state or charge, which can vary. This helps to avoid ambiguity since metals like iron can form ions like Fe2+ or Fe3+.
How do I name a compound with a polyatomic ion?

+
When naming compounds with polyatomic ions, simply write the metal’s name followed by the unchanged polyatomic ion name. For example, CaCO3 is calcium carbonate. If there are multiple polyatomic ions, use prefixes like “di,” “tri,” etc.
Do all ionic compounds have a simple metal-non-metal structure?

+
While many do, ionic compounds can also include polyatomic ions, which are groups of atoms with a net charge that behave as single ions. These complicate the naming somewhat but still follow predictable rules.