5 Essential Tips for Naming Ionic and Covalent Compounds

Understanding Chemical Compound Names

Imagine trying to solve a puzzle where each piece is an atom, and the picture you aim to complete is a molecule. This is the essence of chemistry, where understanding how compounds are named is crucial not only for academics but also for professionals in fields ranging from pharmaceuticals to environmental science. This blog post will delve into the nuances of naming ionic and covalent compounds, providing you with the tools to confidently navigate this complex aspect of chemistry.
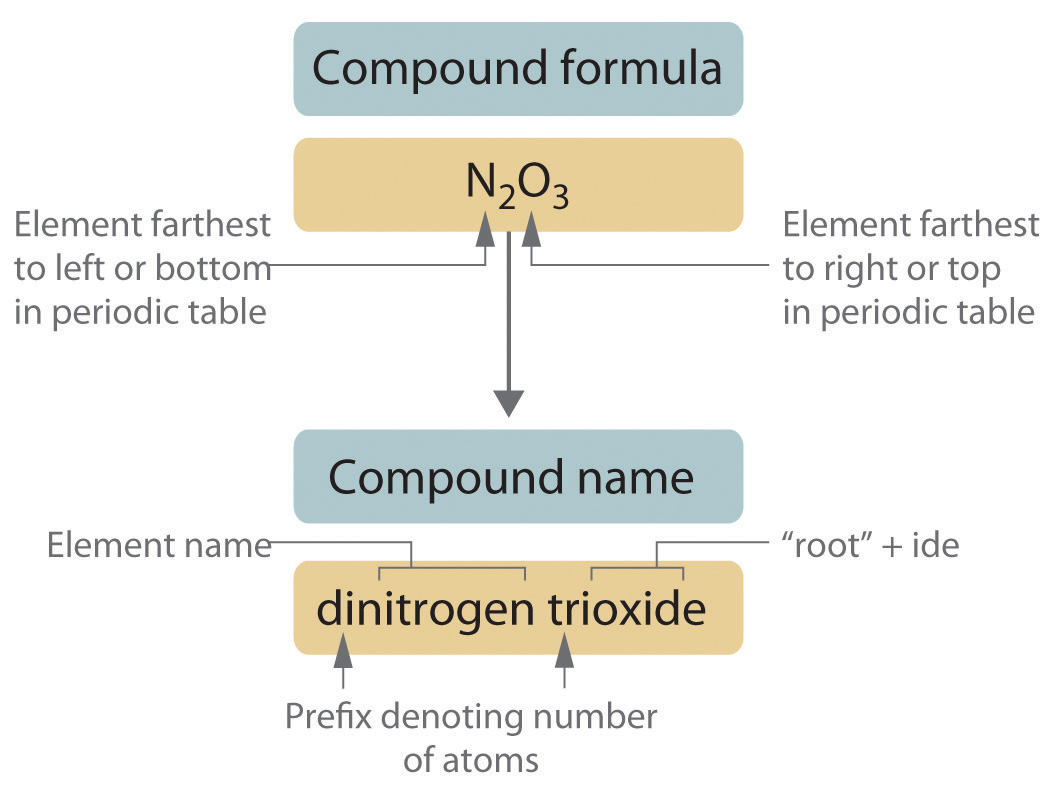
Tip 1: Know Your Prefixes for Covalent Compounds

When dealing with covalent compounds, you often encounter prefixes that denote the number of each atom in the molecule. Here's a simple guide to remember:
- mono- (1)
- di- (2)
- tri- (3)
- tetra- (4)
- penta- (5)
- hexa- (6)
- hepta- (7)
- octa- (8)
- nova- (9)
- deca- (10)
Remember to place these prefixes in order of descending atomic weight. For instance, in sulfur dioxide (SO2), you would write 'sulfur di' and then 'oxide' rather than 'di oxide'.
💡 Note: The prefix 'mono-' is typically omitted for the first element in the compound name unless necessary to clarify its count.
Tip 2: Understand Ionic Charge Rules

Ionic compounds form when one atom donates electrons to another, creating a positive ion (cation) and a negative ion (anion). Here are some key points to remember:
- Metals usually form positive ions. Their charge can often be predicted from their position in the periodic table.
- Non-metals typically form negative ions. For example, chlorine commonly becomes Cl-.
- The charge on the ion should be balanced to maintain electrical neutrality.
Consider the compound Magnesium Chloride (MgCl2). Magnesium, as a group 2 metal, donates 2 electrons, forming Mg2+, while two chlorine atoms each accept one electron, becoming Cl- ions, creating the balance of charges.
🔍 Note: Transition metals can have multiple charges, making it essential to check their oxidation state when naming compounds like iron (Fe). Use Roman numerals to specify the charge in parentheses for these elements.
Tip 3: Use Systematic Naming Conventions

The International Union of Pure and Applied Chemistry (IUPAC) provides systematic names for chemical compounds to avoid confusion. Here’s a quick summary:
- Write the metal or positive ion first, followed by the non-metal or negative ion.
- Use the element name for the metal, with the anion ending in -ide. For example, chloride for chlorine, oxide for oxygen.
- For polyatomic ions, like nitrate (NO3-), the ion's name remains the same in the compound's name.
| Element | Charge | Name When Combined |
|---|---|---|
| Oxygen | -2 | Oxide |
| Nitrogen | -3 | Nitride |
| Chlorine | -1 | Chloride |

🧪 Note: For acids, you'll follow specific naming conventions, like using 'hydro-' for compounds without oxygen or '-ic' and '-ous' for others, reflecting the anion's charge.
Tip 4: Recognize Common Names and Latin Roots
While systematic naming is essential, some compounds have commonly used names:
- Water (H2O)
- Hydrogen Peroxide (H2O2)
- Ammonia (NH3)
- Baking Soda or Sodium Bicarbonate (NaHCO3)
- Table Salt or Sodium Chloride (NaCl)
Understanding these common names can expedite communication in scientific and everyday contexts. Also, recognizing Latin roots can help in deciphering more complex compound names.
Tip 5: Practice and Use Resources

Like any language, chemical nomenclature requires practice:
- Use online nomenclature tools or apps to practice naming.
- Chemistry textbooks often have naming exercises and cheat sheets.
- Participate in forums or study groups to learn from peers.
Consistency in practice will make the process intuitive. Remember, both ionic and covalent naming require understanding of prefixes, charge rules, and systematic conventions.
🧠 Note: Familiarity with the periodic table's trends and common polyatomic ions will save you a lot of time in naming compounds.
Mastering the naming conventions for ionic and covalent compounds not only enhances your understanding of chemistry but also empowers you in various professional fields. Whether you're synthesizing new materials or analyzing water quality, knowing how to name compounds is a fundamental skill. By following these tips, practicing regularly, and using available resources, you'll find yourself confidently navigating the chemical world with ease.
Why is it important to follow systematic naming conventions in chemistry?

+
Systematic naming ensures clarity and prevents misunderstandings in scientific communication. With IUPAC nomenclature, any chemist worldwide can interpret the name of a compound correctly, which is crucial for research, safety, and global understanding of chemical substances.
How do I know if a compound is ionic or covalent?

+
Generally, if a compound consists of a metal and a non-metal, it’s ionic. Covalent compounds form when two non-metals bond together. However, in some cases, especially with transition metals or metalloids, the bonding can be more complex, and electronegativity differences can be considered.
Are there any mnemonic devices to help remember chemical names?

+
Absolutely! Mnemonics like “Queen Bee Eats Nachos Regularly” can help remember element groups or charges. For prefixes, you might use “Monkey Daily Takes Pen Handy Hammer Ought Not Donkey” for mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, deca-.
What if the compound I need to name is not straightforward?

+
In complex cases, you might need to delve into rules for naming hydrates, coordination compounds, or organic molecules. Sometimes, referring to established references or chemical databases is necessary to ensure accuracy.
How do I handle compounds with polyatomic ions in naming?

+
Polyatomic ions have names that remain unchanged when incorporated into a compound’s name. For instance, nitrate in sodium nitrate (NaNO3) keeps the same name as when it’s a standalone ion. Focus on balancing the charges correctly.