5 Essential Tips for Naming Hydrocarbons Easily

Understanding the Basics of Hydrocarbon Nomenclature

Before diving into the strategies to simplify the naming of hydrocarbons, it’s beneficial to revisit the fundamental principles. Naming hydrocarbons involves systematic methods established by the IUPAC (International Union of Pure and Applied Chemistry) to ensure clarity and universality in chemical communication. Here’s a brief overview:
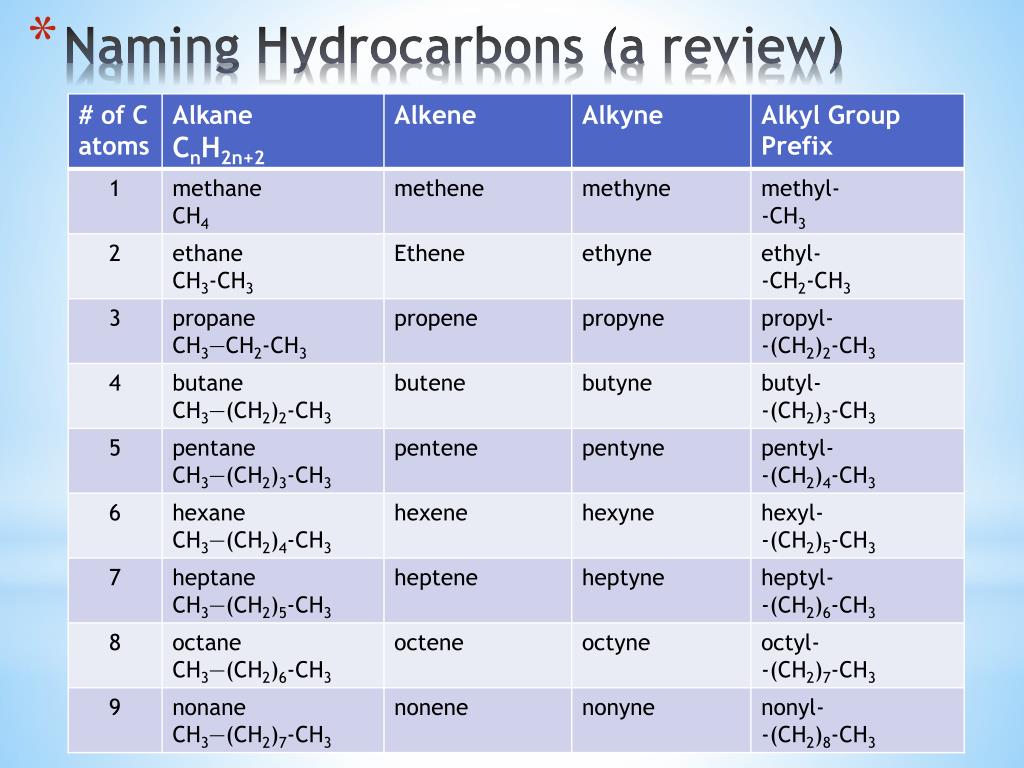
- Acyclic Alkanes: Named by using the Greek prefixes (meth-, eth-, prop-, but-, etc.) corresponding to the number of carbons in the chain, followed by ‘-ane’ indicating a single bond.
- Alkenes and Alkynes: Similar to alkanes, but with the ending ‘-ene’ or ‘-yne’ to indicate double or triple bonds, respectively.
- Cycloalkanes: Rings of carbon atoms are designated with the prefix ‘cyclo-’.
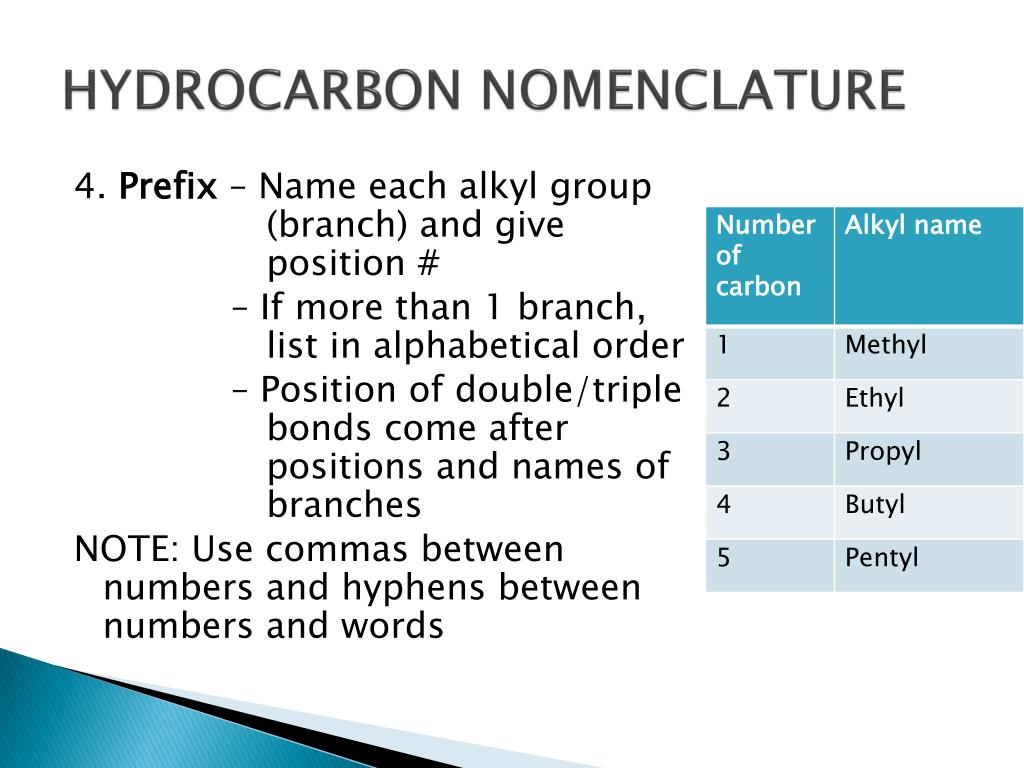
- Branches: Branches or side chains are identified as alkyl groups like methyl, ethyl, etc., prefixed before the main chain’s name.
- Functional Groups: Groups like alcohols (-OH) or carboxylic acids (-COOH) often require an ‘e’ to be dropped from the base hydrocarbon name before the suffix.
Tip 1: Count Carbon Atoms Methodically

The initial step in how to name hydrocarbons effectively is to count the carbon atoms in the longest continuous chain. Here’s how to ensure accuracy:
- Determine the longest chain: Ensure you’re not cutting out any bonds. For example, a molecule with a ‘Y’ structure might fool you into thinking there are fewer carbon atoms than there really are.
- Include branches: Branches are not part of the main chain but must be counted for the total number of carbon atoms.
- Double and triple bonds: These do not alter the carbon count for the chain length.
💡 Note: Always start counting from the end that gives the lower number to the first substituent, which is a rule established for clarity.
Tip 2: Alphabetize Your Branches

When dealing with complex molecules with multiple branches or functional groups, maintaining a clear order is crucial:
- Identify substituents: List all alkyl groups (methyl, ethyl, etc.) or functional groups (halogens, alcohols, etc.) attached to the main chain.
- Alphabetical order: Arrange these groups in alphabetical order, ignoring any numerical prefixes like ‘di-’, ‘tri-’, or ‘tetra-’.
- Naming format: Use commas to separate numbers and hyphens to separate numbers from letters. For example, “3-ethyl-2,4-dimethyl”.
Here is a simple table to show the order:
| Alphabetical Order | Alkyl Group Example |
|---|---|
| 1 | Methyl |
| 2 | Ethyl |
| 3 | Propyl |

Tip 3: Master Prefixes and Suffixes

Understanding and remembering the prefixes and suffixes used in hydrocarbon naming can significantly streamline the process:
Prefixes for carbon atoms: For chains up to 10 carbons, knowing the Greek numerical prefixes can save time:
- 1 - Meth-
- 2 - Eth-
- 3 - Prop-
- 4 - But-
- 5 - Pent-
- 6 - Hex-
- 7 - Hept-
- 8 - Oct-
- 9 - Non-
- 10 - Dec-
Suffixes for bonds:
- Single bond - ‘-ane’
- Double bond - ‘-ene’
- Triple bond - ‘-yne’
Tip 4: Use Tools and Apps to Simplify Naming

In today’s digital age, there are numerous tools and apps designed to assist with chemical nomenclature:
- Chemical Drawing Software: Applications like ChemDraw or MarvinSketch allow you to draw molecules and automatically generate IUPAC names.
- Online Nomenclature Tools: Websites like ChemSpider or PubChem provide search functionality and can name compounds based on their structures.
- Flashcards and Learning Apps: Tools like Anki or Quizlet can help in memorizing prefixes, suffixes, and common names of hydrocarbons.
📲 Note: Using apps and tools for naming hydrocarbons can save time, but ensure you understand the process to avoid dependency.
Tip 5: Practice with Real-World Examples
The most effective way to become proficient in naming hydrocarbons is through practical experience:
- Simulations and Models: Use molecular modeling kits or software to physically or virtually build complex hydrocarbon structures. This helps visualize the molecule and understand its naming.
- Worked Examples: Go through numerous examples, especially of larger and more complex molecules, to apply IUPAC rules in different scenarios.
- Peer Practice: Form study groups or collaborate with classmates to name hydrocarbons together. Explaining your thought process can solidify understanding.
The process of naming hydrocarbons requires a systematic approach, from identifying the longest chain to correctly arranging substituents. By employing these essential tips, you can demystify the nomenclature of hydrocarbons, making it easier to both name and recognize complex chemical structures. With practice and the right tools, the once-daunting task can become a straightforward task in your scientific repertoire.
In this journey of mastering hydrocarbon naming, always remember to revisit and practice the fundamentals. Whether through traditional study methods or modern tools, your consistency will turn these complex molecules into familiar patterns, enhancing your capabilities as a chemist or student of science.
Why is it important to follow IUPAC naming conventions for hydrocarbons?

+
Following IUPAC naming conventions ensures that chemists worldwide can communicate effectively about the same compounds without confusion. It standardizes the naming process, allowing for accurate documentation, chemical research, and education.
Can I use common or trivial names for hydrocarbons?

+
Common or trivial names are often used for simpler hydrocarbons that have historical or general use (like ‘benzene’). However, for more complex or newer compounds, IUPAC names are preferred for precision. It’s good practice to know both where applicable.
How do I handle naming when a molecule has multiple functional groups?
+
In cases of multiple functional groups, prioritize them according to their seniority (alcohol > amine > amide > ester > carboxylic acid > aldehyde > ketone > alkene > alkyne > alkane). Name the compound accordingly, starting from the functional group with the highest priority.
What if I encounter a hydrocarbon that’s too complex to name manually?

+
For very complex hydrocarbons, computer software can be invaluable. These tools use algorithms to identify and name hydrocarbons quickly and accurately, reducing the chance of human error.