Master the Art of Naming Covalent Compounds Easily

Understanding how to name covalent compounds is essential for chemistry students and professionals. These compounds, formed by the sharing of electrons between atoms, have a unique nomenclature that can seem daunting at first but, with the right approach, becomes quite intuitive. Let's explore the rules and strategies for naming these compounds, making the process straightforward and manageable.
Understanding Covalent Bonds

Before diving into the naming conventions, it’s crucial to understand covalent bonds. These bonds occur when atoms share electrons to fill their outer electron shells. This type of bond often forms between nonmetals. Here are some fundamental points:
- Covalent bonds involve sharing electrons.
- They typically form between nonmetals.
- The number of shared electrons defines the bond type; single, double, or triple.
Basic Rules for Naming
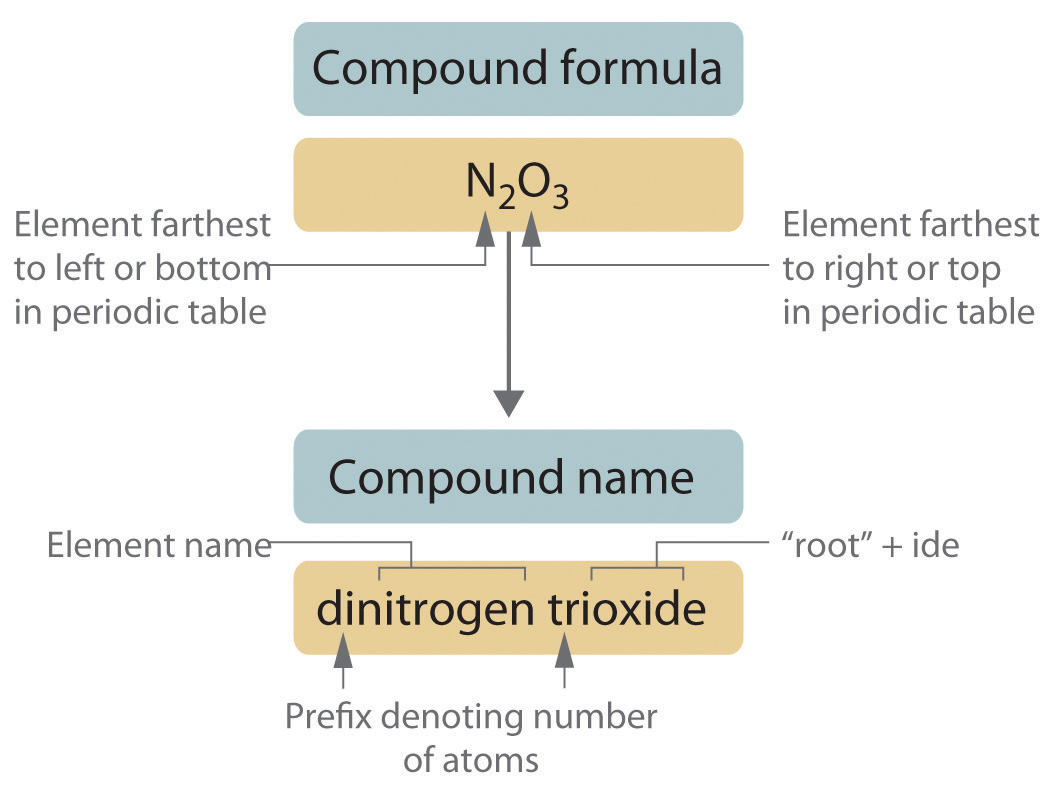
Naming covalent compounds follows these primary guidelines:
- The first element in the formula is named as is.
- The second element is named with an -ide ending.
- Use Greek prefixes to indicate the number of atoms of each element (see table below).
| Number of Atoms | Prefix |
|---|---|
| 1 | mono-* |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |

📝 Note: The prefix 'mono-' is omitted for the first element unless needed for clarity or emphasis.
Practical Examples

Let’s see these rules in action with some common covalent compounds:
- Nitrogen dioxide: NO2
- Carbon tetrachloride: CCl4
- Phosphorus pentachloride: PCl5
Advanced Naming Scenarios

Not all naming situations are straightforward. Here are some scenarios that require additional considerations:
- Compounds with more than one of the same type of atom: For example, SO3 would be named sulfur trioxide, not disulfur trioxide.
- Naming acids: When hydrogen is bonded to another nonmetal, it forms an acid. For binary acids like HCl, you use hydro- + root + -ic acid (hydrochloric acid).
📝 Note: Naming acids can be complex due to different types (binary, oxyacids). Always refer to the appropriate rules based on the compound's composition.
Strategies for Memorization

To master the naming of covalent compounds, consider the following strategies:
- Regular Practice: Use flashcards or quizzes regularly.
- Visual Aids: Create charts or mind maps linking elements with their names and prefixes.
- Relate to Real Life: Think about common substances like water (H2O) and their names for easier memorization.
By applying these techniques and understanding the basic principles, the seemingly complex task of naming covalent compounds becomes much more approachable. Remember, like any language, chemical nomenclature requires practice and patience to master.
In summary, the key to naming covalent compounds involves understanding the rules of bonding, prefixes, and how to apply these rules consistently. With these insights, you're well on your way to becoming adept at chemical nomenclature, which not only aids in your studies but also in communicating scientific concepts effectively.
Why don’t we use ‘mono-’ when there’s just one atom of the first element?

+
The prefix ‘mono-’ is typically omitted for the first element in a formula to keep the naming straightforward. However, it can be used when there’s a need to emphasize the number or avoid confusion.
How do you name covalent compounds with hydrogen?

+
When hydrogen is bonded to another nonmetal, forming a binary acid like HCl, you prefix with ‘hydro-’, followed by the root of the other element and ‘-ic’ acid, hence hydrochloric acid. For oxyacids (containing oxygen), different rules apply.
Can there be covalent compounds with metals?

+
While covalent bonds are most common between nonmetals, some metals in lower oxidation states or with complex ions can form covalent bonds, but these are exceptions rather than the norm.
What is the difference between binary and ternary compounds?

+
Binary compounds contain two different elements, whereas ternary compounds contain three different elements. The naming process changes slightly based on this classification.
How do you memorize Greek prefixes effectively?

+
Flashcards, mnemonics, and associating the prefixes with common words or situations in everyday life can help. Also, practicing with examples will solidify your understanding.