5 Tips for Naming Covalent Compounds Easily

Understanding Covalent Compounds
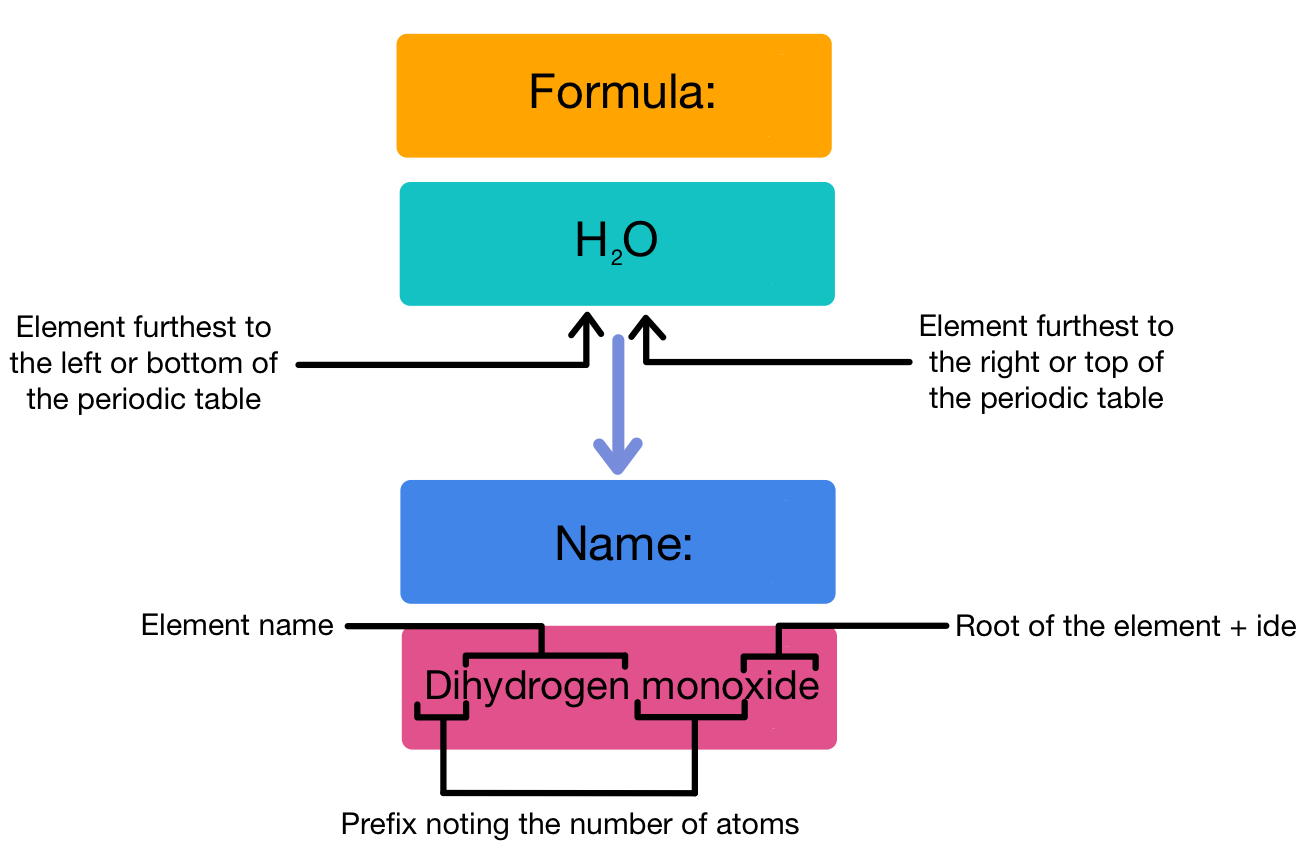
Before diving into the tips for naming these chemical entities, it’s essential to have a clear understanding of what covalent compounds are. Covalent compounds are formed by the sharing of electron pairs between atoms. These bonds are typically found between non-metal elements or non-metal with metalloid atoms, and they tend to exhibit lower melting and boiling points compared to ionic compounds.
The key to naming covalent compounds effectively lies in knowing the prefixes that denote the number of atoms present for each element. Here’s a quick overview:
- Mono - one
- Di - two
- Tri - three
- Tetra - four
- Penta - five
- Hexa - six
- Hepta - seven
- Octa - eight
- Nona - nine
- Deca - ten
Tip 1: Use Prefixes Correctly

The first tip in our naming covalent compounds guide is to correctly use the prefixes that indicate the number of each element within the compound. Here are the steps to follow:
- Identify the number of atoms for each element in the compound.
- Use the appropriate prefix to indicate the number of atoms.
For example, if you have two chlorine atoms and one nitrogen atom in a compound:
- The prefix for nitrogen (1 atom) would be "mono", but this is often omitted in the first element, making the nitrogen part "nitrogen."
- The prefix for chlorine (2 atoms) would be "di", making it "dichloride."
The final name would be nitrogen trichloride.
Tip 2: Order Matters


The sequence in which elements are named in a covalent compound also has specific guidelines:
- The less electronegative element is written first in the formula.
- Element naming order follows the natural placement of elements in the periodic table, from left to right and top to bottom.
For instance:
| Compound | Correct Order | Incorrect Order |
|---|---|---|
| PCl₃ | Phosphorus Trichloride | Trichlorophosphorus |
| NO | Nitrogen Monoxide | Oxygen Mononitride |

⚗️ Note: Oxygen is more electronegative than nitrogen, so the correct order of naming in this case is "nitrogen monoxide".
Tip 3: Understand When to Use -ide Endings

In covalent compounds, the second element always takes an “-ide” ending. Here’s how you apply this:
- When dealing with a binary (two-element) covalent compound, the second element’s name is changed to its -ide form.
Here are examples to illustrate:
- CO₂ would be named carbon dioxide.
- HCl is known as hydrogen chloride.
This practice helps differentiate covalent compounds from ionic compounds in nomenclature.
Tip 4: Avoid Using Prefixed for the First Element in Simple Cases

For simple binary covalent compounds, the prefix “mono” is often omitted from the first element in the name. Here’s how it works:
- In CO₂, you don't say "monocarbon," simply "carbon."
- N₂O would be named dinitrogen monoxide, not "dinitrogen monoxide."
However, when dealing with more complex molecules or in IUPAC nomenclature, the first element may require a prefix if necessary to avoid ambiguity.
Tip 5: Recognize Special Cases and Common Names

There are certain compounds that have common or traditional names which are still used alongside their systematic IUPAC names. Here’s a list of some common exceptions:
- Water (H₂O) - could be named "dihydrogen monoxide" but is almost universally known as "water."
- Ammonia (NH₃) - although its systematic name is "azane."
- Methane (CH₄) - not named using the rules above but known by its unique name.
It’s important to recognize these special cases as they are widely accepted and used in chemistry.
The key to mastering the naming of covalent compounds is to practice these tips frequently. A thorough understanding of the rules will simplify this task, and with time, you’ll find the process of naming these compounds to be quite intuitive.
In summary, naming covalent compounds involves understanding and applying a set of rules and exceptions:
- Use prefixes to indicate the number of atoms of each element.
- Order elements according to electronegativity or periodic table placement.
- Alter the second element’s name to end with “-ide.”
- Omit “mono” for the first element in simple cases, unless necessary for clarity.
- Recognize and use common names for certain well-known compounds.
These tips will not only help you in academic and professional chemistry, but also in everyday understanding of the compounds that make up our world.
What’s the difference between a prefix and a suffix in chemical names?

+
In chemical naming, prefixes indicate the number of atoms in a compound, whereas suffixes are used to denote the type of compound (e.g., -ide for covalent bonds).
Can a covalent compound be named without prefixes?

+
Yes, some common covalent compounds have traditional names and don’t use prefixes (e.g., water, ammonia).
Are there covalent compounds that have an ionic appearance?

+
Yes, compounds like ammonium chloride (NH₄Cl) have ionic bonding characteristics but are still named as covalent compounds due to their molecular nature.



