Naming Binary Compounds Worksheet: Answers Included

In chemistry, understanding how to name binary compounds is crucial for students, researchers, and anyone interested in the chemical sciences. Binary compounds, composed of only two different elements, offer a basic yet fundamental understanding of chemical nomenclature. This article will guide you through the process of naming these compounds, with a detailed worksheet and answers to reinforce your knowledge.
What Are Binary Compounds?

A binary compound is any chemical compound composed of two different elements. These elements can come from any part of the periodic table, but the key is that they form simple, often ionic, structures where one element tends to gain or lose electrons to achieve a stable electron configuration.
Types of Binary Compounds

- Binary Ionic Compounds: These compounds consist of a metal cation and a nonmetal anion. The metal loses one or more electrons to become positively charged, while the nonmetal gains electron(s) to become negatively charged.
- Binary Molecular Compounds: These involve two nonmetals bonding covalently. Here, atoms share electrons to form stable molecules.
Naming Ionic Binary Compounds

The process for naming ionic binary compounds follows these steps:
- Identify the metal (cation) and nonmetal (anion).
- Name the metal first using its standard name.
- Then, name the nonmetal by changing its suffix to “-ide.”
- If the metal can form more than one ion (variable charge), include a Roman numeral after its name to indicate its charge.
⚠️ Note: Remember that some metals like sodium (Na) or potassium (K) have only one common ion, so no Roman numeral is needed.
Naming Molecular Binary Compounds

For molecular compounds, the process involves:
- List the less electronegative element first, followed by the more electronegative element.
- Use Greek prefixes to indicate the number of each type of atom present, except for the first element if it’s only one atom.
- Change the ending of the second element to “-ide.”
Here’s a table to help with Greek prefixes:
| Number | Prefix |
|---|---|
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |

Practice Worksheet
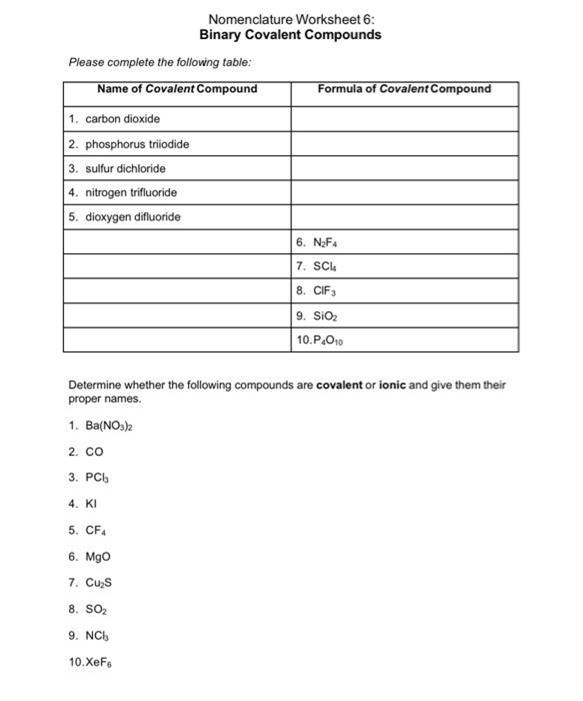
Here’s a worksheet designed to help you practice naming binary compounds. Try to name the following compounds:
- NaCl
- MgF₂
- CuO
- CO₂
- PCl₅
- N₂O
💡 Note: Use the periodic table for reference if needed. Remember that Roman numerals are only used when a metal has variable charges.
Answers to the Worksheet
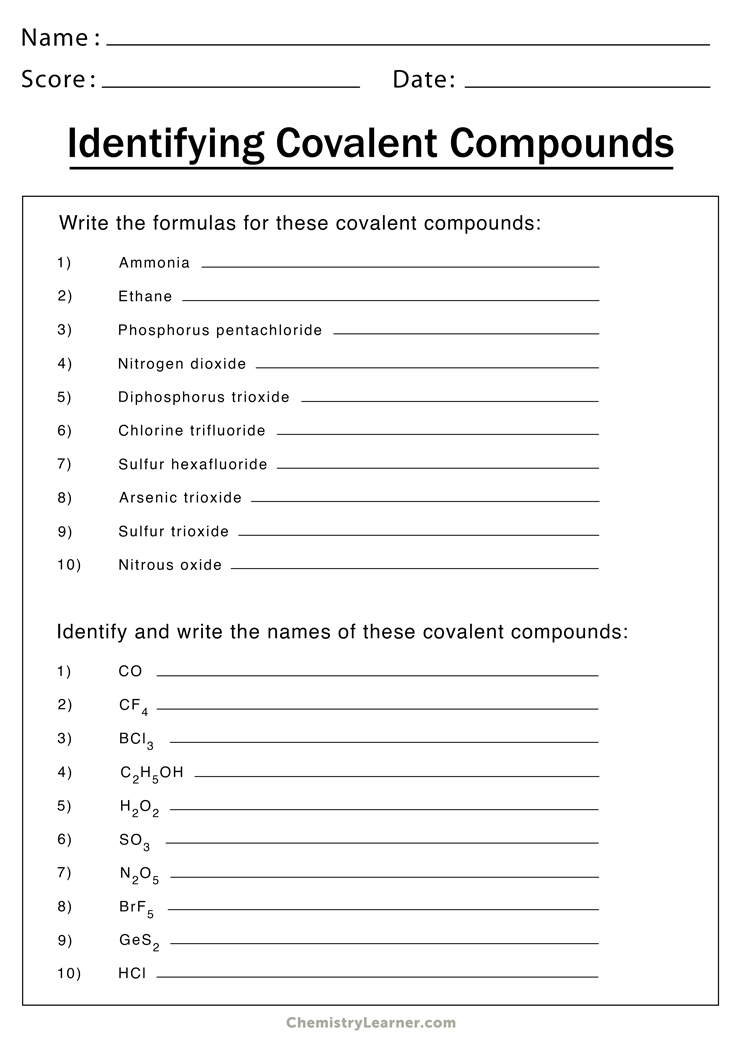
- NaCl - Sodium Chloride
- MgF₂ - Magnesium Fluoride
- CuO - Copper(II) Oxide
- CO₂ - Carbon Dioxide
- PCl₅ - Phosphorus Pentachloride
- N₂O - Dinitrogen Monoxide
Why Naming Matters

Naming compounds correctly is vital for:
- Clarity in communication: Ensures that everyone is talking about the same substance.
- Chemical synthesis: Accurate naming facilitates the correct preparation of compounds in the lab.
- Educational purposes: Understanding nomenclature provides a foundation for more complex chemistry.
Conclusion

As you have now seen, naming binary compounds involves understanding the elements involved, their charges, and the rules of chemical nomenclature. By mastering these rules, you gain not only the ability to communicate effectively with others in your field but also the groundwork necessary to delve deeper into the world of chemistry. Remember, each compound has a unique identity, and by knowing how to name them, you unlock the first key to understanding their properties, reactions, and the very essence of matter itself.
What is the difference between a binary ionic compound and a binary molecular compound?

+
A binary ionic compound consists of a metal and a nonmetal where the metal loses electrons to the nonmetal to form ions. A binary molecular compound, on the other hand, involves two nonmetals sharing electrons in covalent bonds.
Why do some metals not need Roman numerals when naming?

+
Metals like sodium (Na) or potassium (K) only form one common ion, so their charge is predictable and does not need to be specified with a Roman numeral.
Can you name a compound that contains three elements using the same rules?

+
No, these rules apply specifically to binary compounds. Compounds with three or more elements often follow different naming conventions, such as polyatomic ions or ternary compounds.