Mastering Alkane Naming: Your Essential Guide

Alkanes, the simplest form of hydrocarbons, serve as the backbone for understanding organic chemistry. Their systematic nomenclature, established by the International Union of Pure and Applied Chemistry (IUPAC), might seem daunting at first, but with the right approach, you can master the art of alkane naming. This guide will take you through the principles of naming alkanes, ensuring you grasp the fundamentals and apply them effectively.
Understanding Alkane Structures
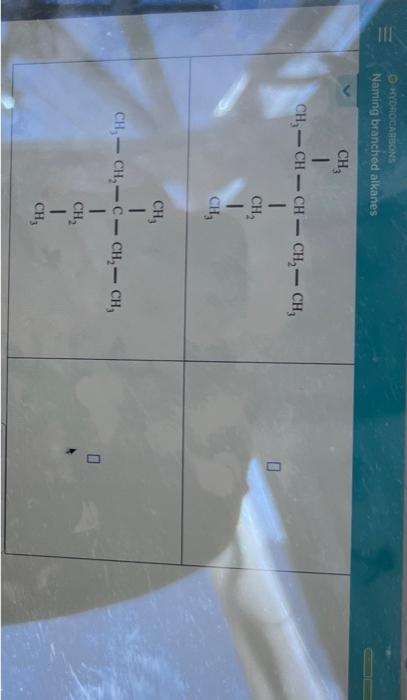
Before diving into nomenclature, it’s beneficial to understand what alkanes are. Alkanes are saturated hydrocarbons where each carbon atom is bonded to four other atoms, following the tetravalency rule. Here’s what you need to know:
- Carbon atoms form chains or rings.
- All C-C bonds are single bonds.
- The molecular formula for an alkane is CnH2n+2 for acyclic alkanes.
📚 Note: The structure of alkanes directly influences their nomenclature, so understanding their basic form is key.
Basic IUPAC Rules for Naming Alkanes

Naming alkanes involves identifying the longest continuous carbon chain, substituents, and applying prefixes and suffixes according to the IUPAC rules:
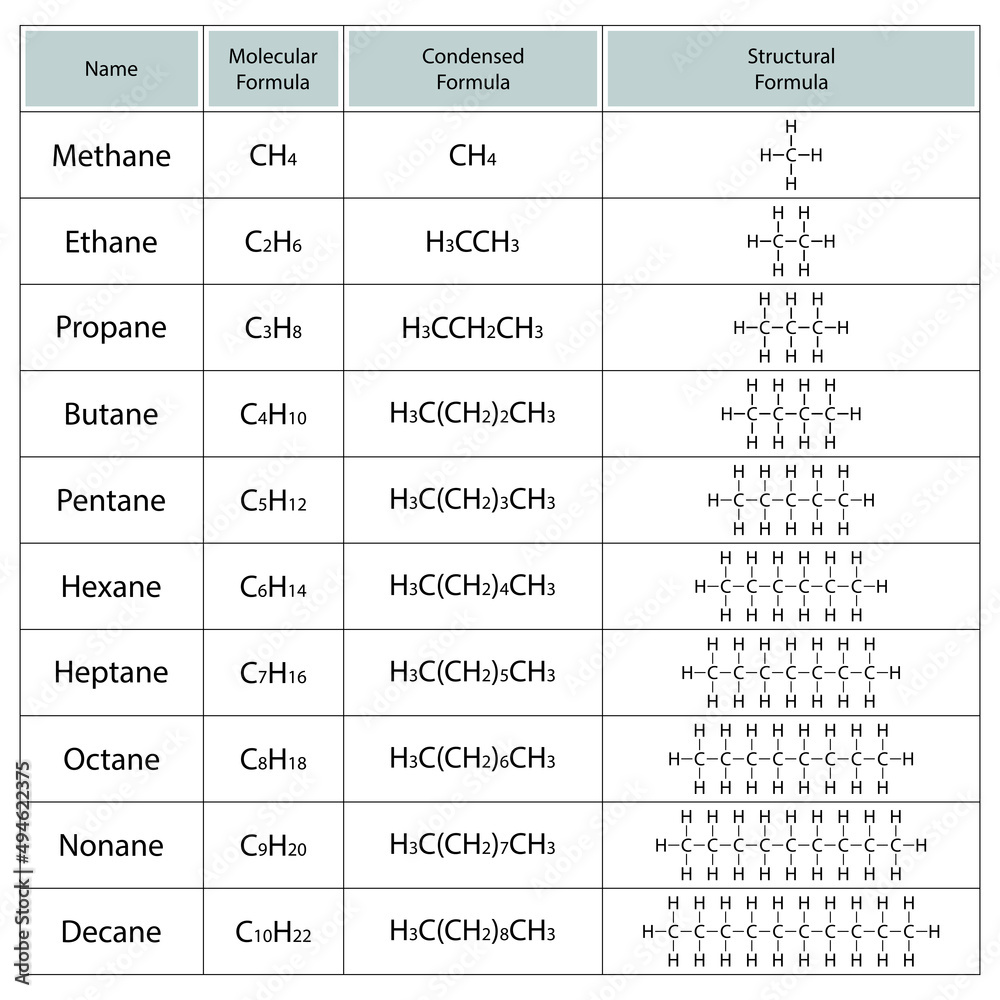
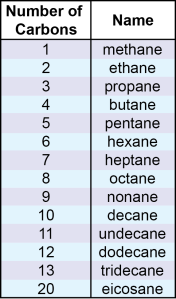
Step 1: Identify the Longest Carbon Chain
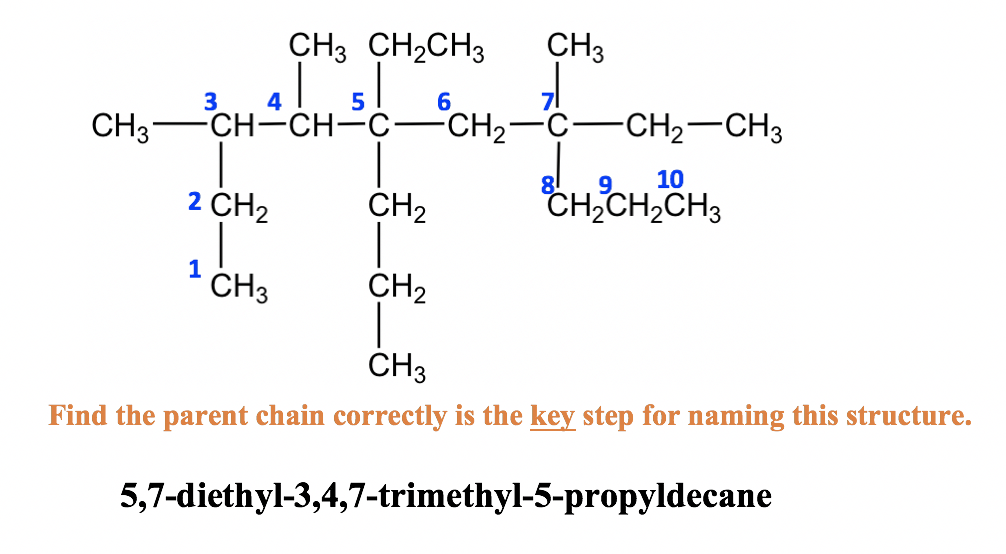
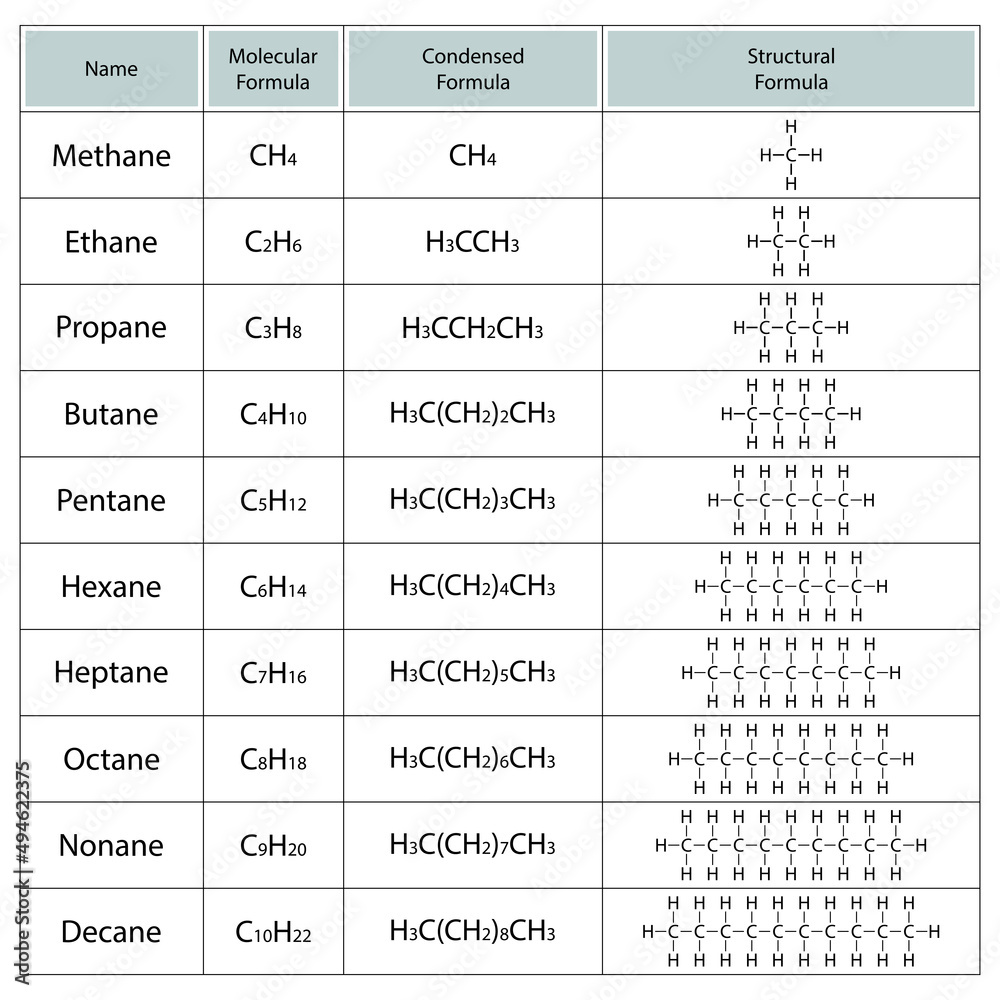
This chain determines the alkane’s parent name. Here are the basic names:
| No. of Carbons | Root Name |
|---|---|
| 1 | Methane |
| 2 | Ethane |
| 3 | Propane |
| 4 | Butane |
| 5 | Pentane |
| 6 | Hexane |

Step 2: Identify and Number the Substituents

- Identify alkyl substituents (branches off the main chain).
- Number the carbons of the main chain so that substituents get the lowest possible numbers.
- Use prefixes like methyl-, ethyl-, propyl-, etc., for branches.
Step 3: Alphabetize and Assemble the Name
Combine the names of substituents, list them alphabetically, and then append the parent name:
- List substituents in alphabetical order.
- Use commas to separate numbers and hyphens to join numbers and substituents.
- When there are multiple substituents of the same type, use prefixes like di-, tri-, tetra-.
More Complex Alkanes
As we venture into naming more complex alkanes, additional considerations come into play:
Cyclic Alkanes

- Use the prefix cyclo- before the parent name.
- Number the ring to assign the lowest possible numbers to substituents.
Alkanes with Branched Chains

- Identify the main chain as before.
- Number substituents so that they receive the lowest combination of numbers.
- For complex branches, name them as if they were independent alkanes, with a prefix ending in -yl.
🧬 Note: For complex molecules, you might need to name secondary chains as a substituent with a number indicating the point of attachment.
Practical Examples of Naming Alkanes

Let’s apply what we’ve learned with some practical examples:
Straight Chain Alkanes
- Example 1: The compound CH3CH2CH3 is propane.
- Example 2: CH3(CH2)3CH3 is pentane.
Branched Alkanes

- Example: CH3-CH2-CH(CH3)-CH2-CH3 is 2-methylpentane.
Cyclic Alkanes

- Example: A ring of five carbons with one methyl group is called 1-methylcyclopentane.
To conclude, mastering the art of naming alkanes according to IUPAC rules involves understanding the basic structure of alkanes, recognizing the longest chain, identifying substituents, and numbering them correctly. These principles not only apply to simple alkanes but also to more complex structures, allowing chemists to communicate clearly and systematically. This proficiency in alkane nomenclature opens the door to understanding more complex organic compounds, enhancing your ability to predict reactions and properties. Remember, practice is key, so take the time to name alkanes regularly, and you'll soon find yourself confidently navigating through organic chemistry nomenclature.
Why is it important to follow IUPAC rules for naming alkanes?

+
IUPAC rules provide a universal system of chemical nomenclature, allowing chemists worldwide to understand and communicate the structure of molecules unambiguously.
Can alkanes have more than one longest chain?
+
No, according to IUPAC rules, there can only be one longest continuous carbon chain which determines the parent name of the alkane.
What if there are two substituents on the same position of a cyclic alkane?
+In cyclic alkanes, if two substituents are on the same carbon, one is considered to be on the lowest possible number, typically 1, and the other on the next available position, often leading to names like 1,1-dimethylcyclopentane.



