Naming Alkanes Made Easy: Practice Worksheet with Answers

In the world of chemistry, the language of hydrocarbons speaks through the structure and names of molecules like alkanes. Understanding how to name these compounds systematically is not only crucial for academic purposes but also has practical applications in industries such as pharmaceuticals, petroleum, and materials science. This blog post will delve into the process of naming alkanes, providing you with a detailed practice worksheet, complete with answers, to solidify your understanding.
Understanding Alkanes

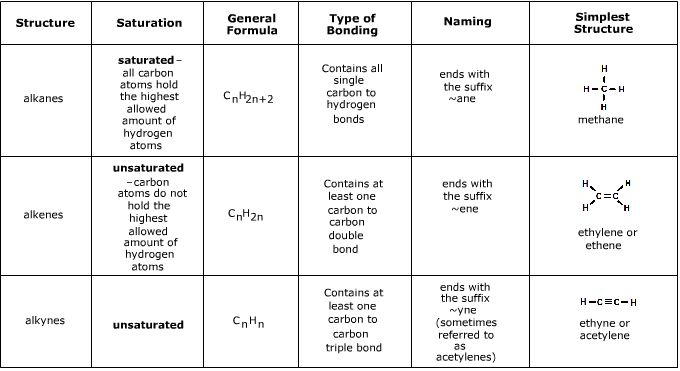
Alkanes are hydrocarbons composed entirely of carbon and hydrogen atoms, connected by single bonds. They are also known as saturated hydrocarbons because they have the maximum number of hydrogen atoms bonded to each carbon atom. Here's a brief overview:
- Basic Structure: Alkanes have a linear structure with no double or triple bonds.
- Nomenclature Rules: These follow the IUPAC (International Union of Pure and Applied Chemistry) guidelines for systematic naming.

Naming Alkanes: A Step-by-Step Guide
Here are the steps you need to follow to name alkanes correctly:
1. Identify the Longest Carbon Chain
Start by finding the longest continuous chain of carbon atoms in the molecule. This number of carbons will be the prefix in the alkane’s name.
2. Count the Carbon Atoms
Count the carbon atoms in the longest chain to determine the base name:
| Number of Carbon Atoms | Name |
|---|---|
| 1 | Methane |
| 2 | Ethane |
| 3 | Propane |
| 4 | Butane |
| 5 | Pentane |
3. Number the Chain
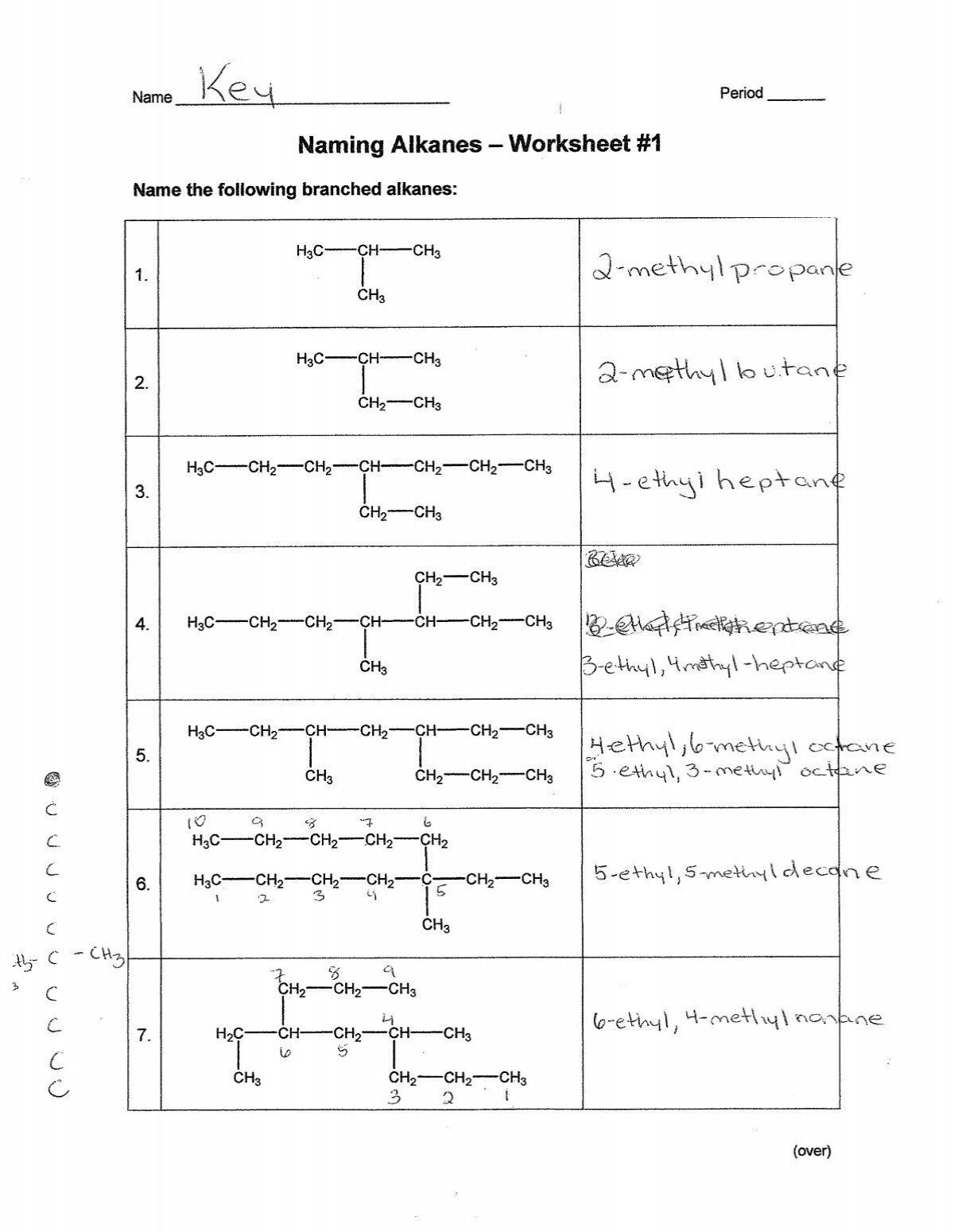
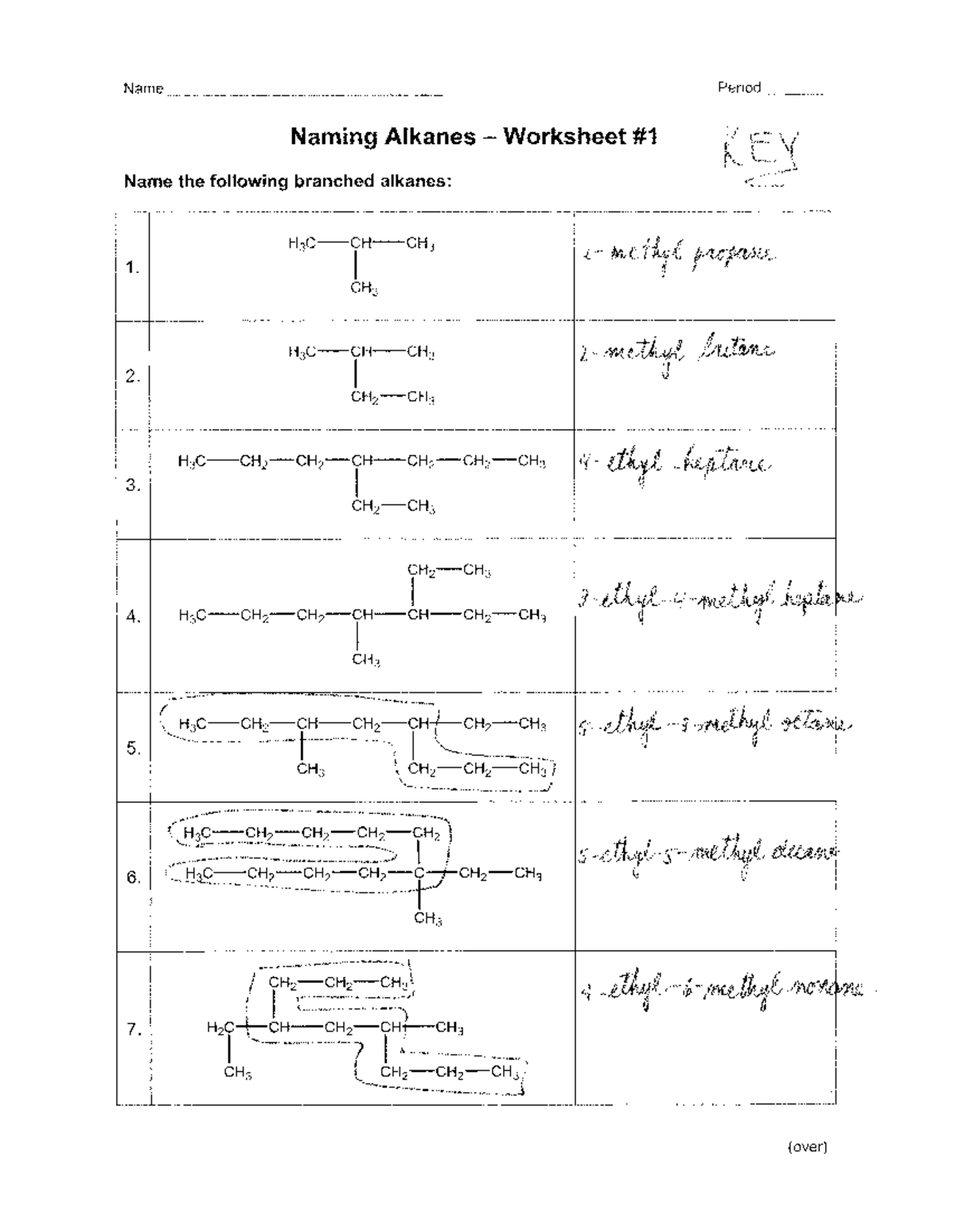
Number the carbon atoms in the chain to give the substituents (branches) the lowest numbers. This often means you’ll start numbering from the end closest to a substituent.
4. Name Substituents
If there are any branches or substituents:
- Use the prefix (methyl-, ethyl-, propyl-) to indicate the substituents.
- Include the location of the substituent by placing the number before the substituent name.
5. Combine All Elements
Write the name in the following order: Substituent names with numbers indicating their positions, followed by the base alkane name.
💡 Note: For alkanes with multiple identical substituents, use prefixes like di-, tri-, tetra- etc., and arrange the substituents in alphabetical order.
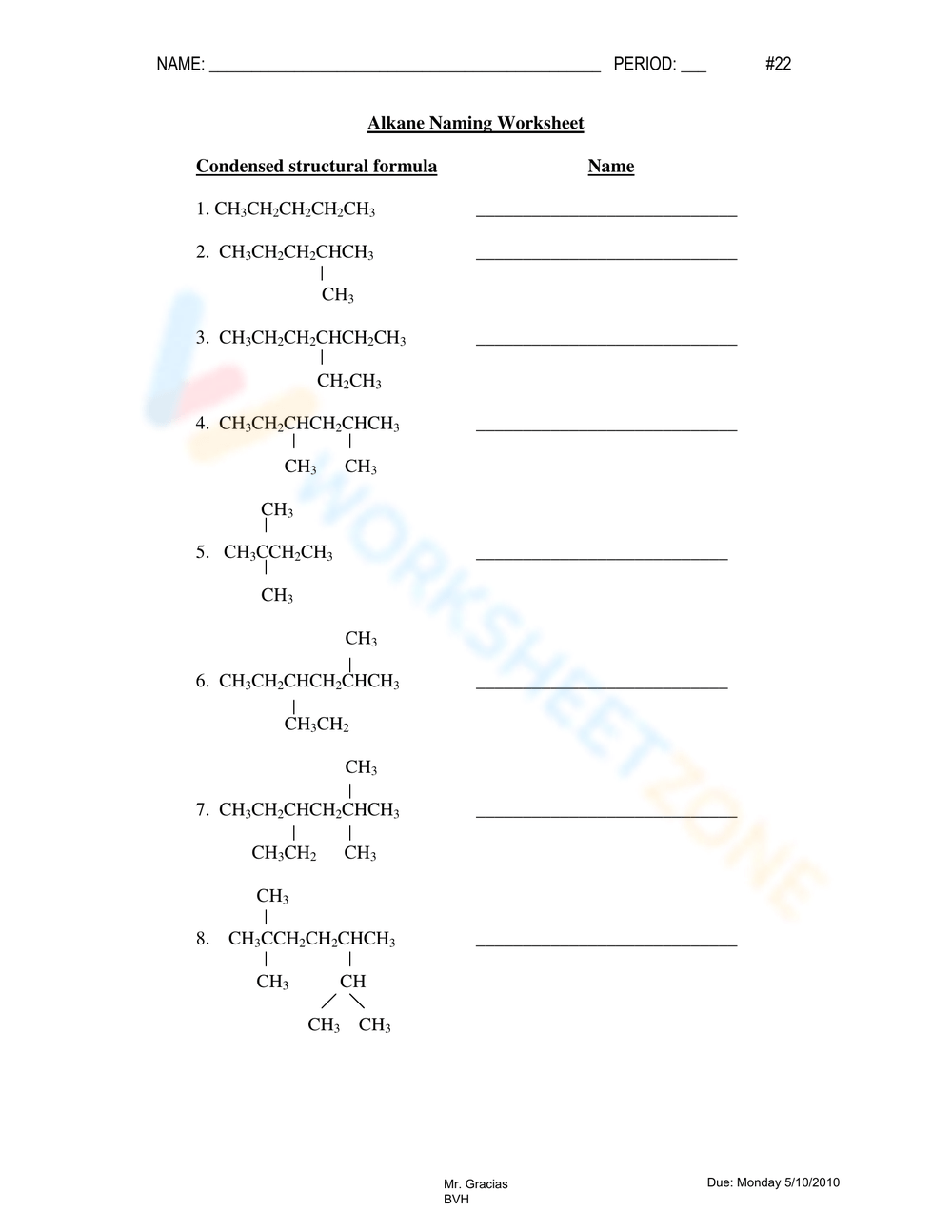
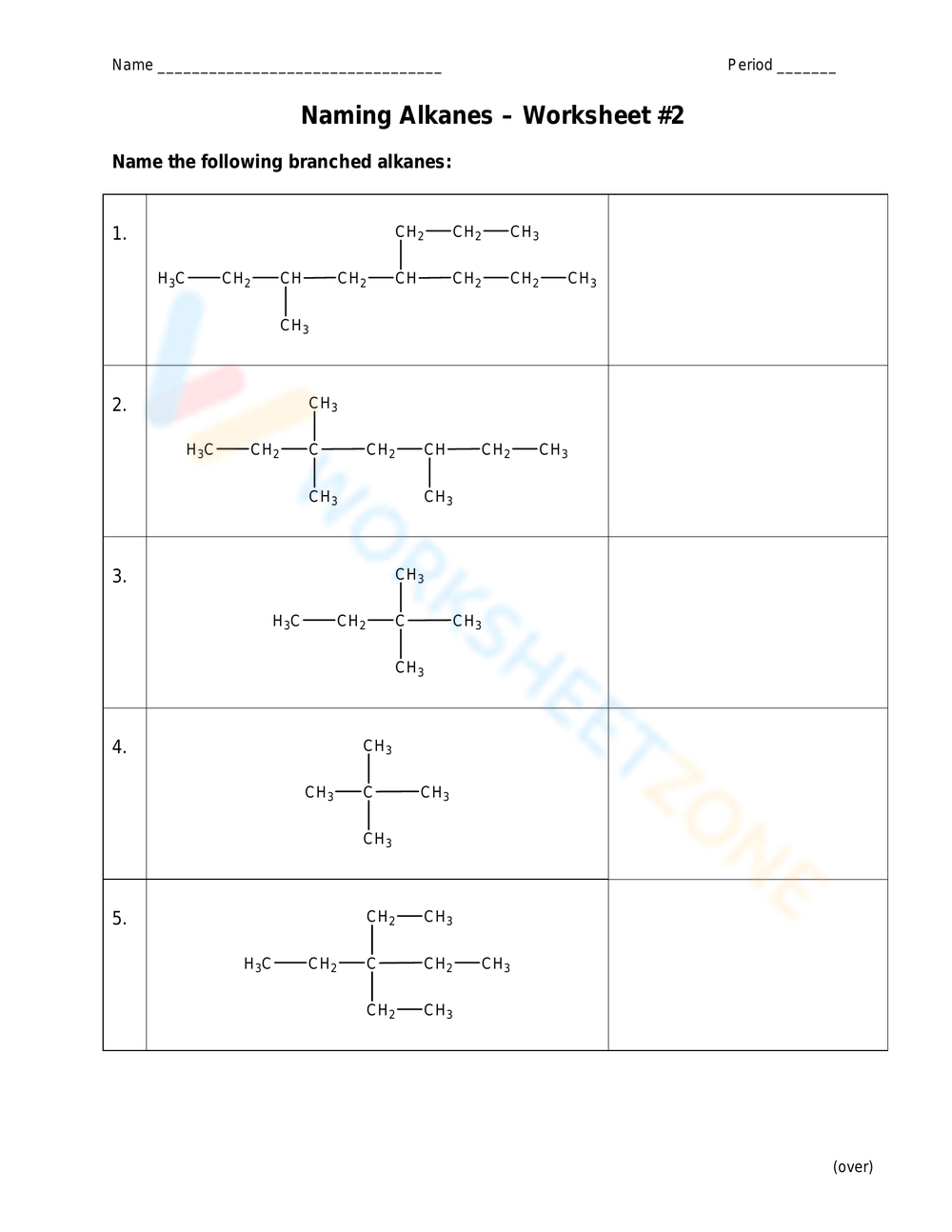
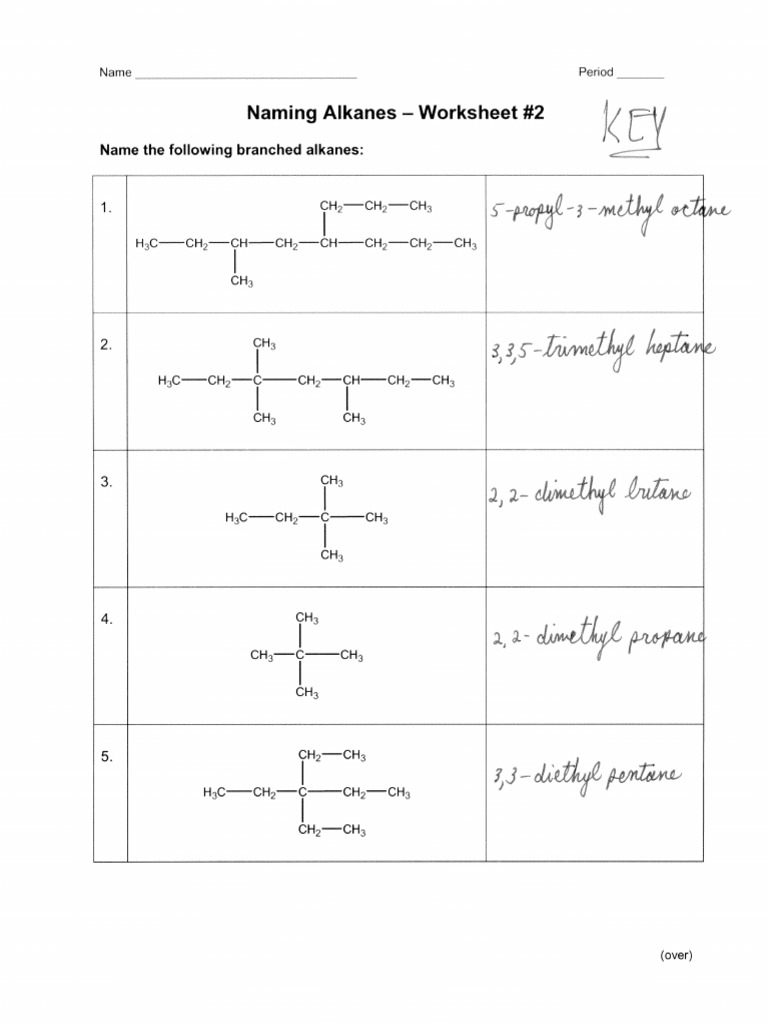
Practice Worksheet with Answers
Let's now apply these rules with a practical exercise:
Example Problems:
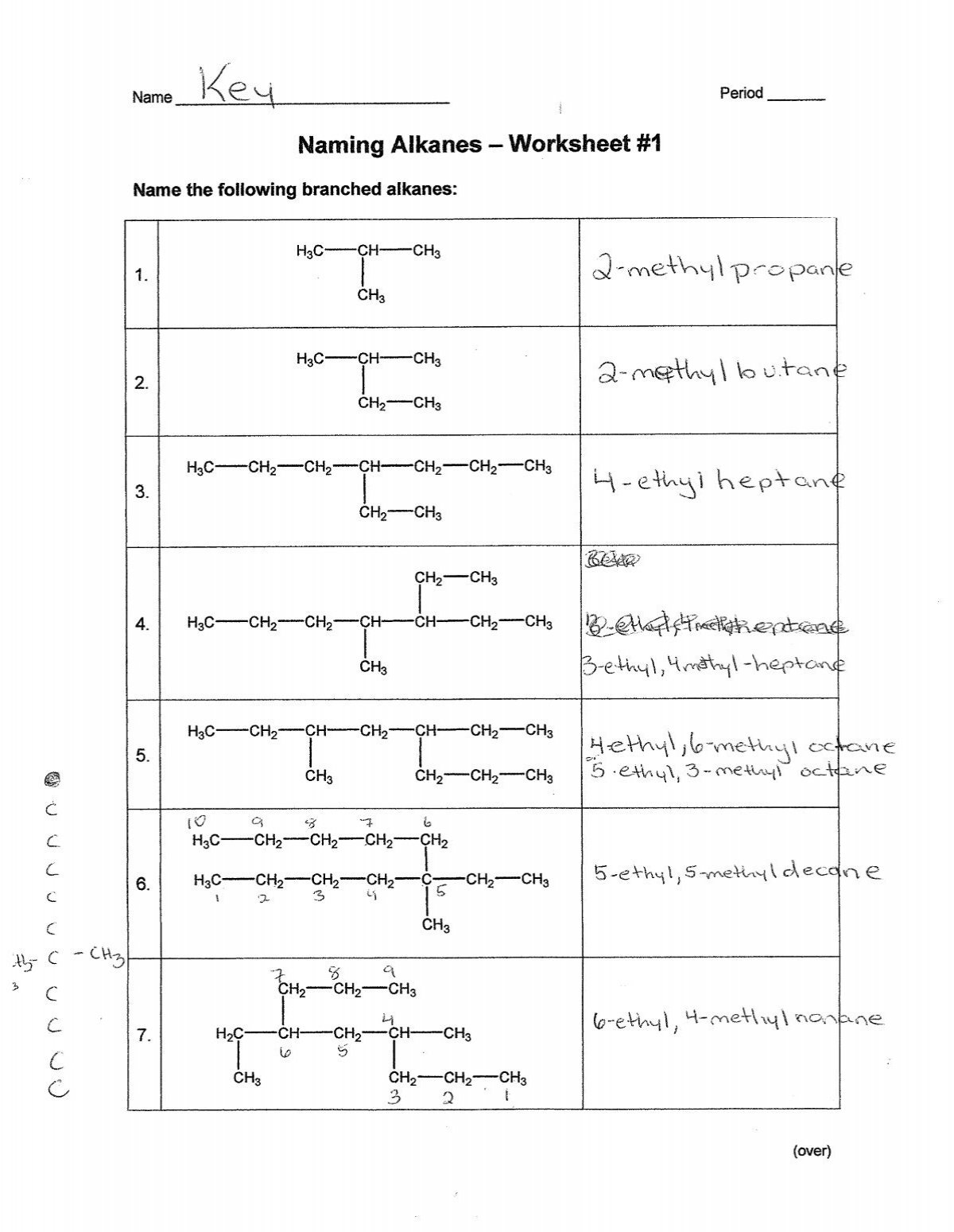
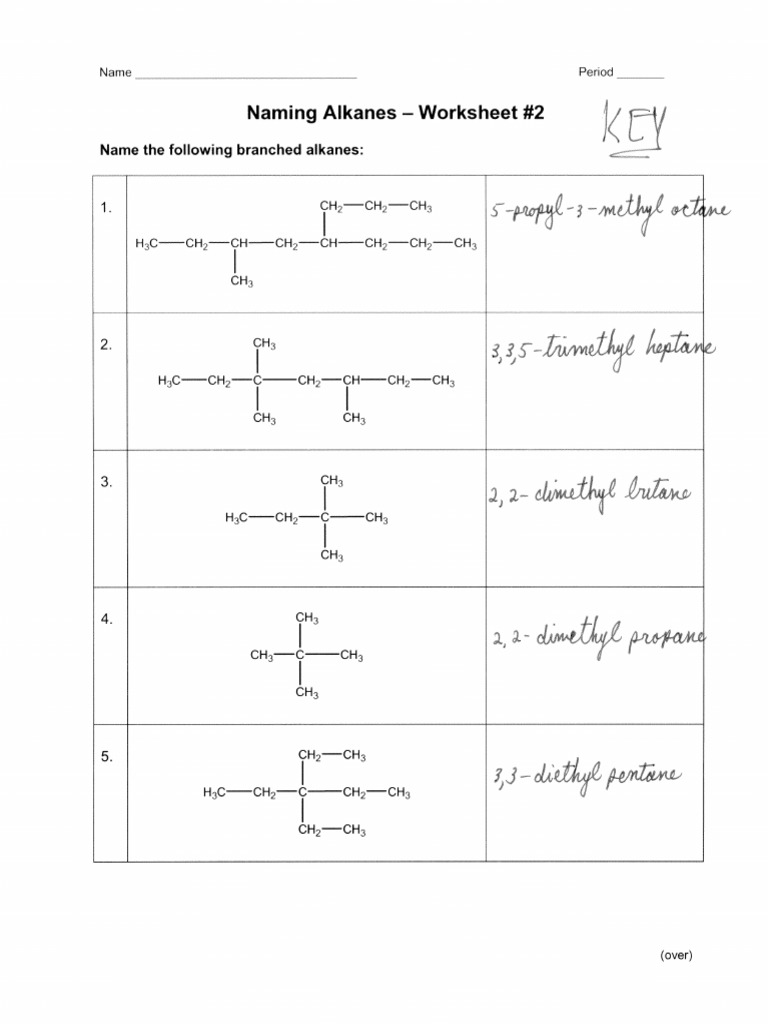
- Problem 1: Draw the structure for 2,3-dimethylpentane.
- Problem 2: What is the IUPAC name for CH3CH(CH3)CH2CH3?
- Problem 3: Name the following alkane:

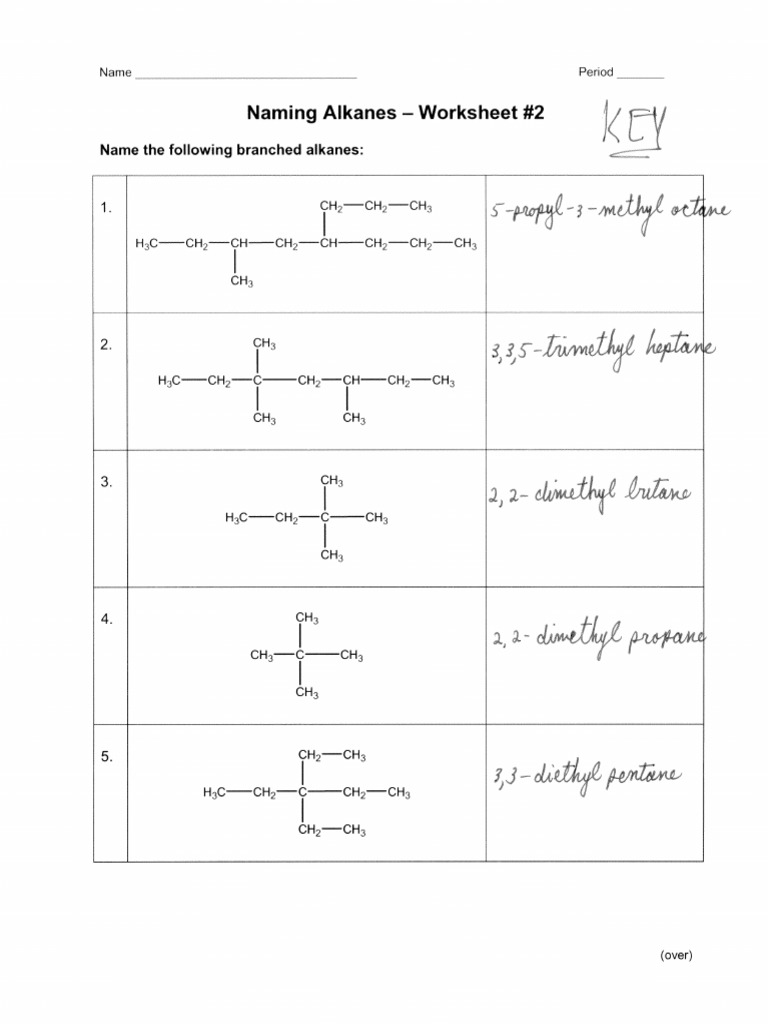
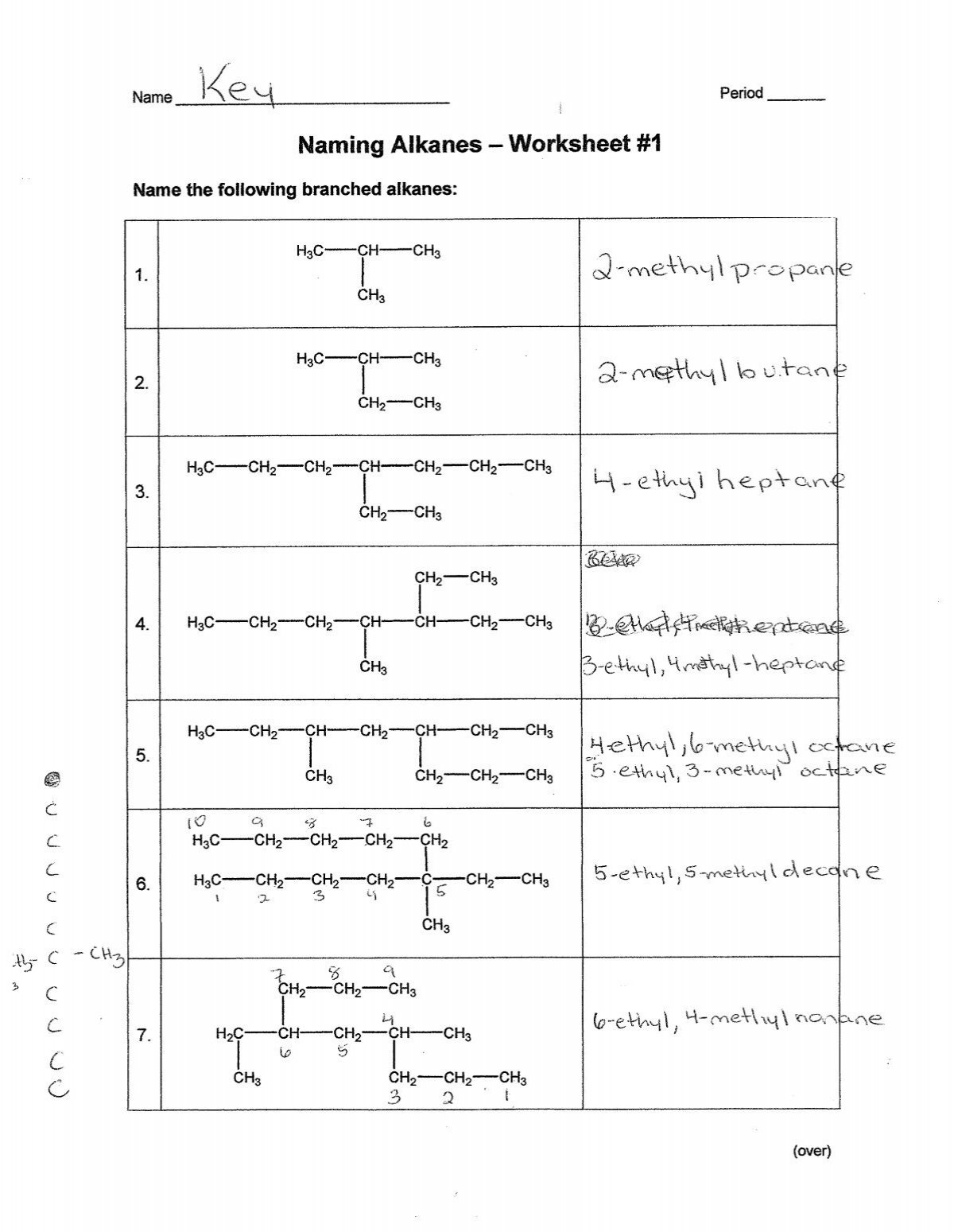
Solutions:
- Solution to Problem 1: Here, pentane (C5H12) has methyl groups at the 2nd and 3rd carbons.
- Solution to Problem 2: This is 2-methylbutane.
- Solution to Problem 3: The alkane shown has a longest chain of 5 carbons (pentane). There are two methyl groups, one at position 2 and one at position 3, making it 2,3-dimethylpentane.
Summary of Key Points

Naming alkanes is an essential skill in chemistry:
- Always start with the longest carbon chain.
- Count carbons to identify the base name.
- Number the chain to minimize the numbers on substituents.
- Name and place substituents correctly.
Why is the numbering system important in naming alkanes?

+
Numbering the carbon chain ensures that substituents get the lowest possible numbers, which reduces confusion and helps in predicting the molecule’s properties.
What if an alkane has a side chain on a side chain?
+
In such cases, name the side chain first, then consider it as a single substituent when naming the entire molecule. The entire substituent group should be enclosed in parentheses if necessary.
How do I name cycloalkanes?
+
Cycloalkanes are named similarly, but you prefix the base alkane name with “cyclo-”. Numbering starts with the first substituent and goes around the ring to keep numbers low.