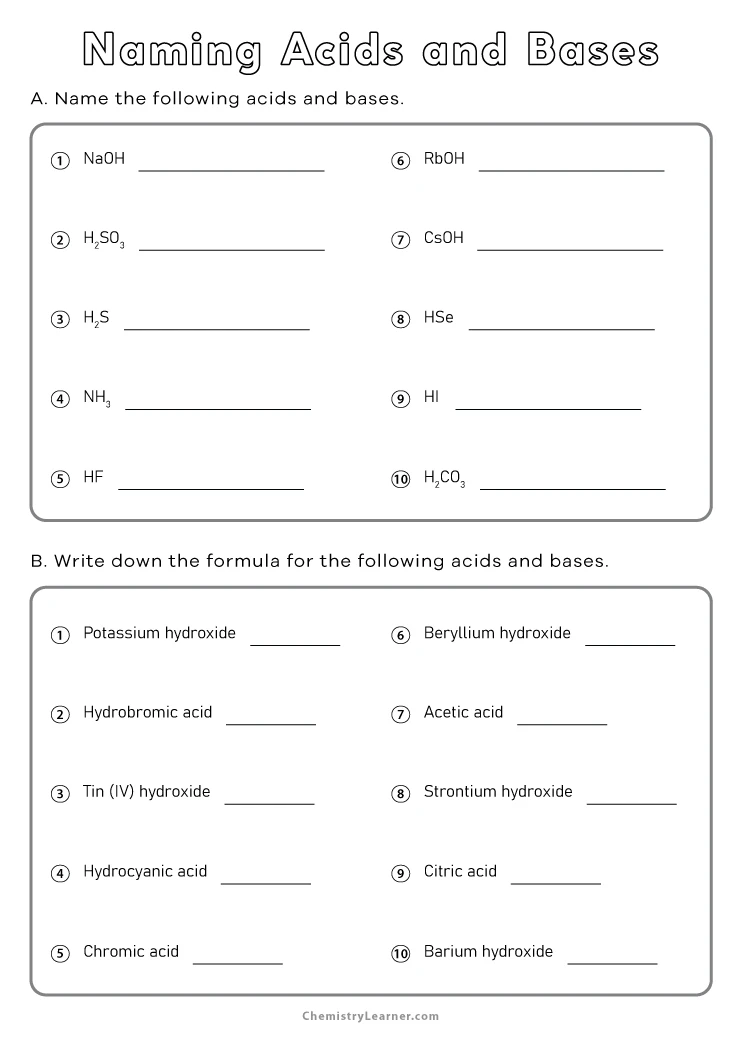
Acid Naming Worksheet: Complete Answers Guide

To effectively work with acids in a laboratory setting or for educational purposes, understanding how to properly name and identify acids is critical. This guide provides an in-depth tutorial on how to name acids based on their anions, ensuring clarity and accuracy in communication and experimental documentation.
Naming Binary Acids

Binary acids are composed of hydrogen and one other element. Here is how to name them:
- Start with the prefix hydro-.
- Follow with the name of the other element with the suffix -ic.
- Finish with the word acid.
For example, HCl (Hydrogen Chloride) when dissolved in water, becomes:
| Formula | Name |
|---|---|
| HCl (aq) | Hydrochloric Acid |
| H2S (aq) | Hydrosulfuric Acid |

Naming Oxyacids (or Oxoacids)

Oxyacids contain hydrogen, oxygen, and another element (usually a non-metal). Here's how to name them:
- Identify the anion and modify its ending:
- -ate suffix becomes -ic (for the acid).
- -ite suffix becomes -ous (for the acid).
- Add the word acid at the end.
Examples:

- Nitrate ion (NO3-) to Nitric Acid (HNO3).
- Nitrite ion (NO2-) to Nitrous Acid (HNO2).
⚠️ Note: When the anion name ends in -ide, we revert to the binary acid naming convention with hydro- and -ic as outlined above.
Complex and Polyatomic Ions

Naming acids from complex polyatomic ions involves understanding the suffix rules for the non-metal anion:
- -ate ions become -ic acids.
- -ite ions become -ous acids.
Here are some examples:
| Formula | Ion | Acid |
|---|---|---|
| HClO4 | Perchlorate | Perchloric Acid |
| HClO3 | Chlorate | Chloric Acid |
| HClO2 | Chlorite | Chlorous Acid |
| HClO | Hypochlorite | Hypochlorous Acid |
Prefixes and Suffixes for Acid Naming:

- Per- (one more oxygen than -ate): Peroxy anion becomes per--ic acid.
- Hypo- (one less oxygen than -ite): Hypo anion becomes hypo--ous acid.
Finally, if the polyatomic ion contains a metal, simply use the name of the metal with the prefix hydr- (without adding any oxygen prefixes or suffixes), followed by acid.
Tips for Recognizing Acid Formulas

- H in front: Look for formulas that start with an 'H', indicating the presence of hydrogen ions.
- Positive Charge: Generally, acids produce positively charged hydrogen ions in water (H+).
- Water Solubility: Acids ionize in water, which can be a key in identifying them.
- Functional Group: Recognize functional groups related to acids such as carboxyl groups (COOH).
This guide should serve as a comprehensive tool for naming acids in various contexts. Whether you are preparing for an exam, working in a lab, or teaching others, the rules above offer a structured approach to understanding and applying acid nomenclature.
In summary, knowing how to name acids involves recognizing key elements and prefixes/suffixes associated with various anions, understanding the differences between binary and oxyacids, and applying these rules systematically.
What is the difference between a binary acid and an oxyacid?
+
Binary acids contain hydrogen and one other non-metal element, like HCl, which becomes hydrochloric acid in water. Oxyacids have hydrogen, oxygen, and another element, like HNO3, which is named nitric acid.
How do I know if an anion ends in -ate or -ite?

+
The anion ending depends on the oxidation state of the central atom in the anion. If it’s in a higher oxidation state, the anion typically ends in -ate. If in a lower state, it ends in -ite.
What is the rule for acids from ions with ‘Per-’ or ‘Hypo-’ prefixes?

+
‘Per-’ indicates one more oxygen atom than in the -ate ion, making the acid name start with ‘per-ic’ (like perchloric acid). ‘Hypo-’ indicates one less oxygen than in the -ite ion, leading to the acid name starting with ‘hypo-ous’ (like hypochlorous acid).