5 Tips for Mastering Molecular Polarity Worksheets

Understanding molecular polarity is crucial for students studying chemistry, especially when it comes to excelling in worksheets that test this knowledge. Molecular polarity affects a variety of chemical properties including solubility, boiling points, and reactivity. Here are five essential tips to master molecular polarity worksheets effectively:
1. Understand the Basics of Polarity


Before diving into complex worksheets, you need a solid grasp of what molecular polarity is:
- Polar Bonds: Arise when there is a significant difference in electronegativity between bonded atoms.
- Polar Molecules: Result from an asymmetric distribution of electron density or from the presence of polar bonds arranged asymmetrically.
- Electronegativity: The ability of an atom to attract shared electrons in a covalent bond.
2. Recognize Common Functional Groups
Functional groups can directly influence the polarity of a molecule:
| Functional Group | Polarity |
|---|---|
| Hydroxyl (-OH) | Polar |
| Aldehyde (-CHO) | Polar |
| Amine (-NH2) | Polar |
| Halogen (-X) | Often Increases Polarity |

⚠️ Note: Not all functional groups will make a molecule polar; this depends on the overall molecular geometry and the presence of other polar or nonpolar groups.
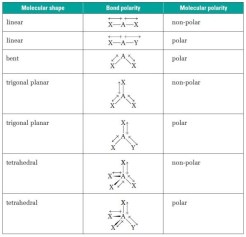
3. Use Molecular Geometry to Determine Polarity

Molecular shape plays a critical role in determining polarity:
- Linear Molecules can be polar if there are different atoms bonded to the central atom, like in HCl.
- Bent Molecules are often polar due to asymmetrical electron distribution, e.g., H2O.
- Tetrahedral Molecules like CH4 are nonpolar, but if one or more atoms are replaced with different atoms, it can become polar (e.g., CH3Cl).
- Trigonal Planar can be polar if there is an electronegative atom bonded to the central atom, e.g., BF3 (which actually isn’t polar due to symmetry, but helps illustrate the concept).
4. Practice Determining Polarity with Sample Exercises

Work through a variety of molecular polarity exercises:
- Identify bond types and assess electronegativity differences.
- Draw Lewis structures to visualize the arrangement of atoms.
- Determine molecular geometry using VSEPR theory.
- Apply the concept of symmetry to conclude on molecule’s polarity.
Here’s an exercise for practice:
- Molecule: CO2.
- Is it polar? No, due to its linear shape and symmetrical distribution of polar bonds.
- Bond types: Two polar C=O bonds.
- Molecular Geometry: Linear.
📝 Note: Regular practice with different molecular structures will help you become proficient in identifying polar and nonpolar molecules quickly.
5. Review Key Concepts with Online Resources and Textbooks

To solidify your understanding:
- Online Videos and Tutorials: Look for resources that explain molecular polarity visually.
- Interactive Simulations: Use molecular modeling software to experiment with molecule shapes.
- Textbooks: Review chapters on electronegativity, bond polarity, and molecular geometry.
In wrapping up these tips, remember that mastering molecular polarity isn’t just about understanding concepts but also applying them through repeated practice. By focusing on the electronegativity, molecular geometry, and symmetry, you can predict the behavior of compounds in chemical reactions and physical properties. Keep exploring, practicing, and connecting the dots between theory and its practical applications to excel in molecular polarity worksheets.
Why is understanding molecular polarity important?

+
Understanding molecular polarity is crucial for predicting how substances will interact in chemical reactions, how they dissolve in solvents, and how they behave in physical changes like boiling or freezing.
How can I tell if a molecule is polar or nonpolar?

+
Look at the electronegativity differences between bonded atoms and consider the molecular geometry. If the molecule has a net dipole moment due to asymmetrical electron distribution, it’s polar.
Can a molecule be polar if it has all nonpolar bonds?

+
A molecule cannot be polar if all its bonds are nonpolar. Polarity requires an asymmetry in electron distribution, which can’t be achieved with nonpolar bonds alone.