Unlock Answers: Molecular Geometry Worksheet Guide

Understanding the complex world of chemistry often starts with grasping the fundamentals of molecular geometry. This guide will take you through the essential concepts needed to confidently approach any molecular geometry worksheet. Let's embark on this learning journey together.
Why Molecular Geometry Matters


Before diving into the specifics, let’s consider why molecular geometry is important:
- Understanding Reactivity: The shape of a molecule directly influences how it interacts with other molecules, which can predict chemical reactions.
- Impact on Physical Properties: Geometry affects properties like polarity, solubility, and boiling point.
- Influence on Biological Processes: Many biological processes depend on the specific geometry of enzymes and substrates.
Key Concepts for Molecular Geometry

Here are the primary elements you should know:
- VSEPR Theory: Valence Shell Electron Pair Repulsion Theory helps predict the geometry based on the repulsion of electron pairs.
- Electron Geometry vs. Molecular Geometry: Electron geometry considers all electron pairs, while molecular geometry looks only at the arrangement of atoms.
- Lone Pairs: These non-bonding pairs of electrons significantly influence molecular shape.
How to Determine Molecular Geometry
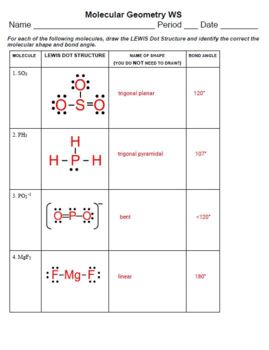
Let’s walk through the steps to determine molecular geometry:
- Count Valence Electrons: Sum the valence electrons of all atoms in the molecule.
- Draw the Lewis Structure: Use the total valence electrons to sketch the arrangement of atoms.
- Determine Electron Pairs: Count all electron pairs (bonding and non-bonding).
- Apply VSEPR: Consider how the pairs would arrange to minimize repulsion.
- Analyze Geometry: Use the resulting pattern to decide the molecular shape.
📝 Note: For molecules with lone pairs, the molecular geometry often differs from the electron geometry due to the greater repulsion by lone pairs.
Common Molecular Geometries

| Number of Electron Groups | Number of Lone Pairs | Molecular Geometry |
|---|---|---|
| 2 | 0 | Linear |
| 3 | 0 | Trigonal Planar |
| 3 | 1 | Bent |
| 4 | 0 | Tetrahedral |
| 4 | 1 | Trigonal Pyramidal |
| 4 | 2 | Bent |
| 5 | 0 | Trigonal Bipyramidal |
| 5 | 1 | See-saw |
| 5 | 2 | T-shaped |
| 6 | 0 | Octahedral |

Practical Examples of Molecular Geometry

Let’s look at some real-world examples:
- CO2 (Carbon Dioxide): Linear geometry leads to non-polarity.
- H2O (Water): Bent geometry results in polarity due to the bond angle and lone pairs.
- NH3 (Ammonia): Trigonal pyramidal shape, slightly polar due to lone pair influence.
💡 Note: Understanding the shape of water is crucial for explaining its high boiling point and ability to form hydrogen bonds.
In summary, mastering molecular geometry involves learning how to predict and understand the three-dimensional structure of molecules. This knowledge not only enhances your performance in chemistry but also provides insights into molecular behavior, chemical reactions, and biological phenomena. By systematically applying the VSEPR theory and recognizing common geometries, you can decode the molecular world, answering questions posed by worksheets or even everyday observations of how substances interact.
What is the difference between electron geometry and molecular geometry?

+
Electron geometry considers the arrangement of all electron pairs (both bonding and non-bonding), while molecular geometry looks only at the positions of the atoms themselves, excluding the electron pairs.
How does the presence of lone pairs affect molecular geometry?

+
Lone pairs exert greater repulsive force than bonding pairs, causing the molecule to adopt a shape that minimizes this repulsion. For example, a molecule with one lone pair on the central atom can distort from tetrahedral to trigonal pyramidal or bent.
Can molecular geometry influence chemical reactivity?

+
Yes, the shape of a molecule determines the accessibility of electron pairs for bonding, affecting how a molecule will react with others. For instance, the bent geometry of water allows for strong hydrogen bonding, influencing its reactivity.
What are the limitations of the VSEPR model?
+
VSEPR provides a simplified model and does not account for all factors like pi bonding, the effect of d-orbitals, or the intricacies of crystal structures. It’s an approximation useful for predicting basic molecular shapes.