Molecular Geometry Worksheet Answer Key Revealed
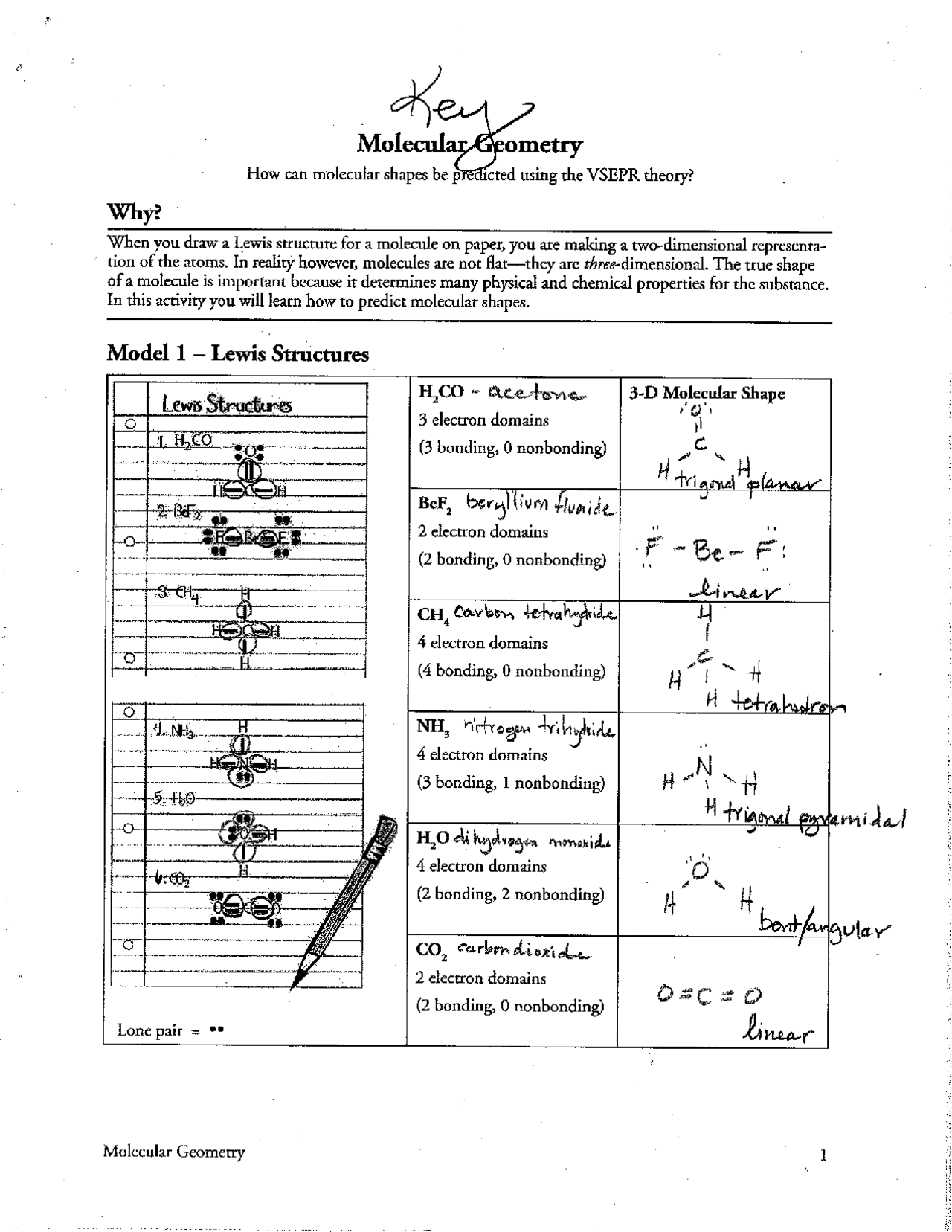
Understanding molecular geometry is essential in the realm of chemistry, particularly when predicting how molecules will behave in various chemical reactions or physical interactions. This post will delve into the molecular geometry worksheet answers, providing students and educators with a comprehensive guide to the common shapes of molecules, their angles, and their hybridizations.
Understanding Molecular Geometry

Molecular geometry refers to the spatial arrangement of atoms in a molecule, determined by the valence shell electron pair repulsion theory (VSEPR). This model posits that electron pairs around a central atom repel each other, leading to specific geometric shapes:
- Linear: Two atoms bonded to the central atom at 180 degrees.
- Trigonal Planar: Three atoms around the central atom at 120 degrees apart.
- Tetrahedral: Four atoms bonded at angles of 109.5 degrees.
- Trigonal Bipyramidal: Five atoms with angles of 120 degrees in the plane and 90 degrees between the axial bonds.
- Octahedral: Six atoms at 90 degrees to each other.
Key Factors Influencing Geometry

Several factors influence molecular geometry:
- Electron Domain Geometry: The arrangement of all electron pairs around the central atom.
- Lone Pair Repulsion: Lone pairs take up more space than bonding pairs, often changing the ideal geometry.
- Hybridization: Mixing of atomic orbitals to form new, hybrid orbitals suitable for bonding.
Worksheet Answer Key: Common Molecular Shapes

| Molecular Formula | Shape | Angle | Hybridization |
|---|---|---|---|
| BeCl2 | Linear | 180° | sp |
| BF3 | Trigonal Planar | 120° | sp2 |
| CH4 | Tetrahedral | 109.5° | sp3 |
| PCl5 | Trigonal Bipyramidal | 90°, 120° | dsp3 |
| SF6 | Octahedral | 90° | d2sp3 |

Interpreting Molecular Geometries

When students work through molecular geometry worksheets, here are some critical points to consider:
- Count Electron Pairs: Including both bonding and non-bonding electrons to determine the electron domain geometry.
- Polarity: Molecules can be polar or nonpolar based on their symmetry and the presence of lone pairs.
- Electronegativity: Influences bond polarity and affects molecular shape through steric effects.
💡 Note: Always consider lone pairs in your geometry; they significantly affect the shape by repelling other electron domains.
Practical Applications of Molecular Geometry

The study of molecular geometry isn’t just academic; it has numerous practical applications:
- Catalysis: The shape of catalyst surfaces can enhance reaction rates.
- Biochemistry: Understanding protein folding and enzyme function.
- Pharmacology: Drug design relies heavily on molecular shape to interact with biological targets.
Understanding molecular geometry provides a three-dimensional view of molecules, which is critical for predicting their reactivity, interactions, and properties. This knowledge not only aids in academic studies but also has far-reaching implications in the industrial, medical, and environmental fields. By mastering the principles outlined in molecular geometry worksheets, students gain valuable insight into the behavior of matter at its smallest scale, laying the foundation for advanced studies and innovations in chemistry.
Why is molecular geometry important in chemistry?

+
Molecular geometry is crucial because it dictates how molecules interact with each other, influencing properties like solubility, polarity, and reactivity, which are fundamental to chemical processes.
What causes molecules to adopt certain shapes?

+
Electron repulsion is the primary force; electron pairs, whether bonding or non-bonding, repel each other and thus arrange in a way to minimize this repulsion, leading to specific geometries.
How do lone pairs affect molecular geometry?

+
Lone pairs take up more space than bonding pairs, pushing other electron domains closer together, which can distort the ideal molecular geometry into shapes like trigonal pyramidal or bent.
Can a molecule have multiple correct geometries?

+
While a molecule has one most stable geometry, it can adopt less stable, but still possible, geometries due to thermal energy, known as dynamic effects or in excited states.
What’s the difference between electron domain geometry and molecular geometry?

+
Electron domain geometry considers all electron pairs, while molecular geometry only considers the arrangement of atoms around the central atom, excluding lone pairs.