Molecular Geometry Worksheet: Master Shapes Easily

Understanding molecular geometry is essential for students of chemistry. It provides insights into how atoms are arranged in three-dimensional space, which influences a molecule's physical and chemical properties. This blog post will guide you through mastering molecular geometry with a detailed worksheet, explaining various shapes and their significance.
What is Molecular Geometry?

Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It's determined by the valence shell electron pair repulsion (VSEPR) theory, which predicts the spatial distribution of atoms based on the minimization of electron-pair repulsions:
- Linear: Two atoms bonded to a central atom with 180° angles between them.
- Trigonal Planar: Three atoms bonded to a central atom, all in one plane, with bond angles of 120°.
- Tetrahedral: Four atoms bonded to a central atom, creating a pyramid-like structure with bond angles of 109.5°.
Worksheet: Explore Molecular Shapes
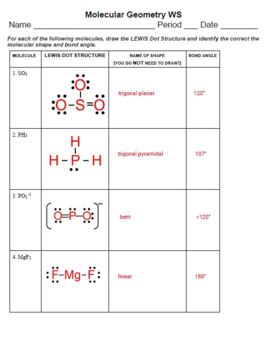
Let's dive into a worksheet that will help you master molecular shapes:
Exercise 1: Identifying Shapes
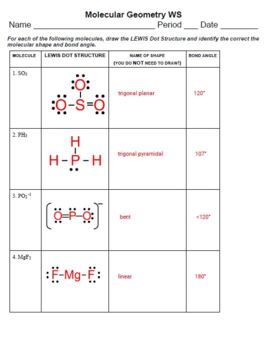
| Molecule | Number of Electron Groups | Shape |
|---|---|---|
| CO2 | 2 | Linear |
| BCl3 | 3 | Trigonal Planar |
| NH3 | 4 | Trigonal Pyramidal |
| CH4 | 4 | Tetrahedral |
| H2O | 4 | Bent (Angular) |

Exercise 2: Predicting Geometry

Use the VSEPR theory to predict the molecular geometry of the following compounds:
- BeCl2
- BH3
- CH2O
⚠️ Note: Remember that lone pairs, though they are not part of the bond angles, do contribute to the electron group count, which affects the geometry.
Real-World Applications of Molecular Geometry

Understanding molecular geometry has real-world implications:
- Pharmacology: Drug molecules' effectiveness can be influenced by their shape, determining how they fit into enzyme active sites.
- Catalysis: The shape of a catalyst determines its efficiency in facilitating chemical reactions by providing an environment for reactants to interact.
- Molecular Electronics: The configuration of electrons in molecules plays a role in designing nanoscale electronic devices.
Limitations and Considerations
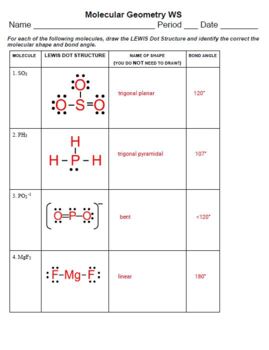
Molecular geometry has its limitations:
- It does not predict the strength or nature of chemical bonds.
- Complex molecules might have geometries that are not easily approximated by simple VSEPR models.
- Resonance structures and delocalized electrons can affect the molecule's shape.
Having traversed the realm of molecular geometry through our comprehensive worksheet, we hope you've developed a more nuanced understanding of this critical aspect of chemistry. From predicting how a molecule will interact with others to understanding its stability and reactivity, molecular geometry is the cornerstone. Remember that mastering molecular geometry involves both theory and practice. Continue to engage with this topic, as it will serve as a foundational pillar in your chemistry studies, leading to further explorations into more advanced topics like stereochemistry and crystal structures.
What is the significance of bond angles in molecular geometry?

+
Bond angles are crucial as they indicate the spatial arrangement of atoms within a molecule. They affect the molecule’s shape, which can influence its polarity, reactivity, and physical properties like melting and boiling points.
Can molecular geometry change with temperature?

+
While molecular geometry is largely determined by electron repulsion, temperature changes do not significantly alter the shape of individual molecules. However, temperature can affect molecular vibrations, which might slightly distort geometries at high temperatures.
How does lone pair repulsion influence molecular geometry?

+
Lone pairs exert greater repulsion than bonding pairs. This leads to a reduction in bond angles in molecules, altering their shape. For example, water (H2O) has a bent geometry because the lone pairs push the bonding pairs away, reducing the H-O-H bond angle to less than 109.5°.



