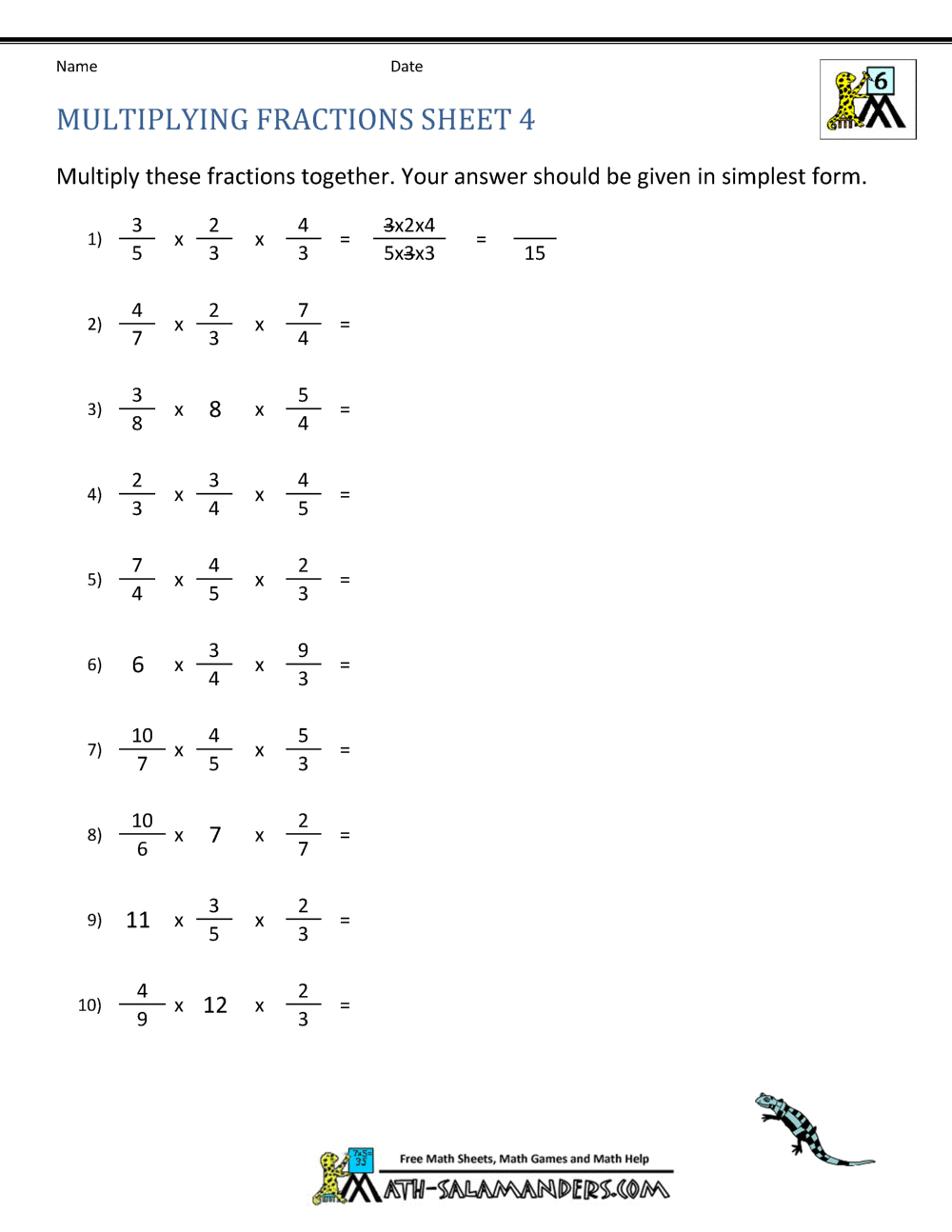Master Molecular and Empirical Formulas with Our Worksheet

Mastering molecular and empirical formulas can unlock a deeper understanding of chemical composition, making it an invaluable skill for students and professionals in chemistry alike. This comprehensive worksheet dives into the essentials of both molecular and empirical formulas, offering step-by-step guidance to help you excel in chemical formula mastery.
Understanding Molecular Formulas

The molecular formula of a compound indicates the actual number of atoms of each element in a single molecule of the compound. Here’s how you can comprehend and calculate molecular formulas:
- Identify the Elements: First, list down the elements present in the compound.
- Find Mass Percentages or Ratios: Determine the mass percentage or the ratio of elements in the compound through experiments or given data.
- Calculate the Empirical Formula: Use the mass percentage to find the smallest whole number ratio of atoms, leading to the empirical formula.
- Determine the Molecular Mass: If known, use the molecular mass to convert the empirical formula to the molecular formula by multiplying each atom ratio by the same number to match the molecular mass.
- Example: Given: Compound has C 54.5%, H 9.1%, and O 36.4% by mass. Calculate its molecular formula.
- Convert percentages to grams for a 100g sample.
- Find the moles of each element.
- Calculate the simplest ratio by dividing each mole by the smallest mole value.
- If molecular mass is given (e.g., 180g/mol), multiply empirical formula units to match this mass.
Calculating Empirical Formulas

The empirical formula provides the simplest whole number ratio of atoms in a compound, which is key in understanding the basic composition without knowing the actual molecule structure:
- Mass to Mole Conversion: Convert the mass of each element to moles using their atomic masses.
- Find the Ratio: Divide each element's moles by the smallest moles to get the simplest ratio.
- Round to Whole Numbers: Adjust ratios to whole numbers, rounding when necessary, to achieve the empirical formula.
- Example: Given: 0.441g H, 0.357g N, and 1.143g O. Find the empirical formula.
- Convert mass to moles.
- Divide by smallest value (11.43 for H, 11.48 for N, 11.43 for O).
- Get a ratio of 1H:1N:1O, so empirical formula is HNO.
🧪 Note: When dealing with elements that have very close or identical ratios, rounding can sometimes lead to slight errors. Be cautious and double-check your results.
Worksheet Practice

| Problem | Empirical Formula | Molecular Formula |
|---|---|---|
| A compound contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen. | C: 3.33, H: 6.66, O: 3.33 (Rounded to C: 3, H: 6, O: 3) | If molecular mass is 90g/mol, the molecular formula is C3H6O3. |

To optimize your learning experience:
- Work through the examples provided in the worksheet.
- Use real-life applications and data to relate formulas to tangible compounds.
- Engage in group discussions or ask for feedback on your calculations.
🔍 Note: Always verify your calculated formula against experimental or known data where possible. This not only validates your work but also reinforces your learning through comparison.
By mastering both molecular and empirical formulas, you'll develop a robust foundation in understanding chemical composition. Whether you're deciphering the ingredients in pharmaceuticals, studying the environment, or simply curious about the world around you, these concepts are universally applicable and profoundly insightful. With regular practice and the tools provided in this worksheet, you're well on your way to becoming proficient in these fundamental aspects of chemistry.
What’s the difference between empirical and molecular formulas?

+
An empirical formula shows the simplest whole-number ratio of atoms in a compound, whereas the molecular formula indicates the actual number of atoms of each element in one molecule.
How do you calculate molecular mass?
+
Calculate the molecular mass by summing the atomic masses of all the atoms in a molecule as indicated by the molecular formula.
Can the molecular formula be the same as the empirical formula?

+
Yes, for compounds where the simplest ratio (empirical formula) is also the actual number of atoms in the molecule (molecular formula), they are the same. For example, water (H2O) has the same empirical and molecular formulas.