Mole Worksheet Chemistry Answers: Your Ultimate Guide

Introduction to Mole Concepts in Chemistry

Understanding the mole concept in chemistry is fundamental to anyone delving into the subject. The mole serves as a bridge between microscopic entities (atoms, molecules, ions) and macroscopic measurements (grams, liters). This guide aims to simplify the complex, often intimidating, world of mole calculations and provide clear, step-by-step answers to common questions.
What is a Mole in Chemistry?

In chemistry, a mole (mol) is defined as the amount of substance containing as many elementary entities as there are atoms in 12 grams of pure carbon-12. This number, approximately 6.022 x 1023, is known as Avogadro's number. Here are some key points about moles:
- Moles relate the number of particles in a substance to its mass.
- One mole of any substance contains Avogadro's number of entities.
Mole Calculations and Conversions

Mole calculations often involve converting between moles, mass, number of particles, and molar mass. Here are the fundamental steps:
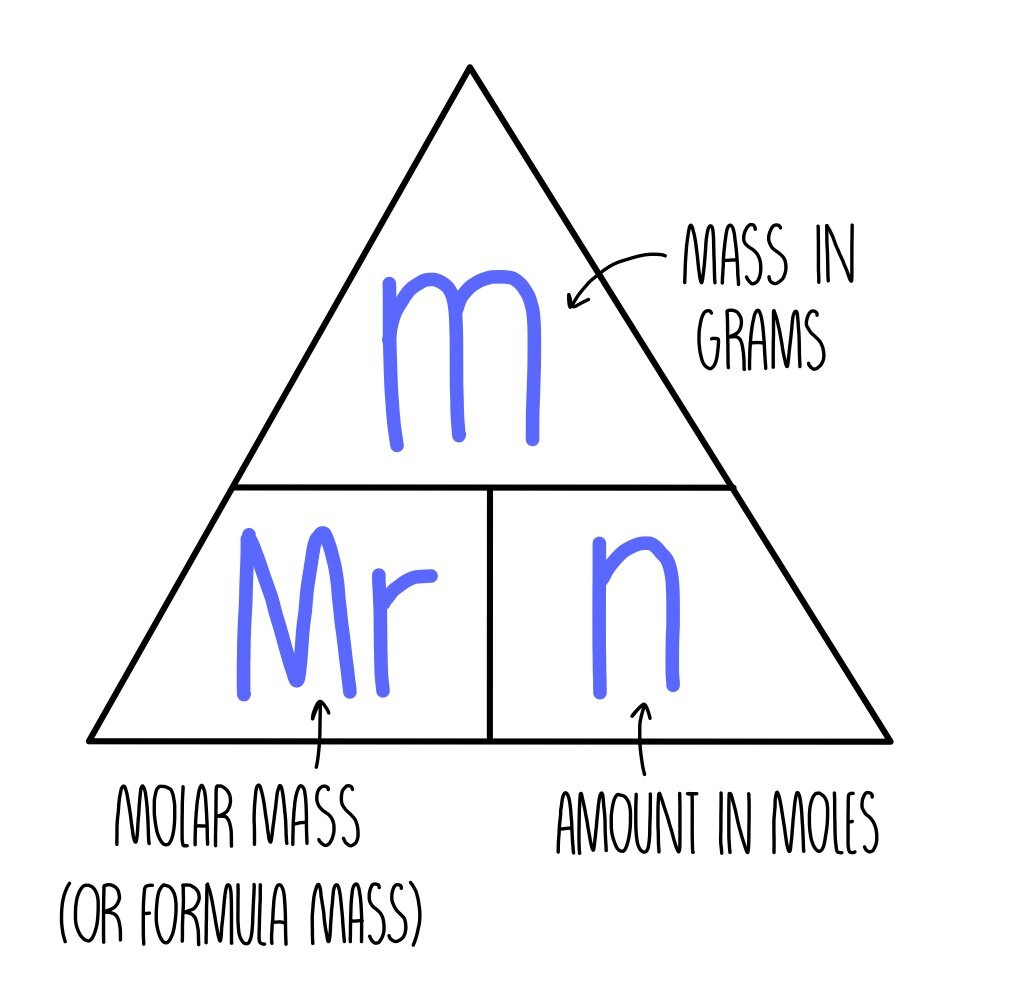
Converting Moles to Mass

To find the mass of a substance given the number of moles:
Molar mass (g/mol) x Number of Moles = Mass (g)
- Find the molar mass of the substance from the periodic table.
- Multiply by the number of moles.
Converting Mass to Moles

To find the number of moles given the mass:
Mass (g) / Molar mass (g/mol) = Number of Moles
- Divide the mass by the molar mass to get moles.
Converting Moles to Number of Particles

To find the number of particles from moles:
Number of Moles x Avogadro's Number = Number of Particles
- Multiply the number of moles by Avogadro's number (6.022 x 1023).
Converting Number of Particles to Moles

To find moles from the number of particles:
Number of Particles / Avogadro's Number = Number of Moles
- Divide the number of particles by Avogadro's number.
Practical Applications of Moles

The mole concept is not just theoretical; it has practical applications in:
- Stoichiometry: Helps determine how much of each reactant is needed and what amount of product will form.
- Laboratory Measurements: Useful for measuring exact quantities of substances in experiments.
- Pharmaceutical Industry: Critical for drug formulation and dosage calculations.
Common Mistakes in Mole Calculations

Here are some common pitfalls when dealing with mole calculations:
- Confusing molecular and molar masses.
- Using incorrect units (e.g., confusing grams with kilograms).
- Forgetting to use Avogadro's number in particle-to-mole conversions.
🧪 Note: Molar mass refers to the mass of one mole of a substance in grams. Ensure you use the correct periodic table values for your calculations.
Advanced Mole Topics

Mole Ratios

Mole ratios are crucial when dealing with chemical reactions. They are derived from the coefficients of a balanced chemical equation:
| Reactants | Products | Ratio (Reactants:Products) |
|---|---|---|
| 2 H2 (g) | O2 (g) | 2:1 |
| 2 H2 (g) | 2 H2O (l) | 1:1 |

Empirical and Molecular Formulas

Empirical formulas give the simplest whole number ratio of atoms in a compound, while molecular formulas show the actual number of atoms:
- Empirical formula: C2H4
- Molecular formula: C2H4 or C6H12 (for cyclohexane)
In this in-depth guide, we've explored the mole concept, its applications, and common calculations. By mastering these fundamentals, students and professionals can navigate the complexities of chemistry with greater ease, making precise measurements and predictions in both the lab and theoretical work. Remember, the key to success with moles lies in understanding the relationships between the mass of a substance, its volume, and the number of particles it contains.
What is the difference between a mole and Avogadro’s number?

+
The mole is a unit of amount of substance, while Avogadro’s number is the amount of entities (atoms, molecules, etc.) in one mole of any substance. Essentially, Avogadro’s number is a constant used to relate moles to the number of particles.
How do I calculate the molar mass of a compound?
+
Calculate the molar mass by adding the atomic masses of each element in the compound. Use the periodic table to find the atomic masses and multiply by the number of atoms of each element present.
Why is understanding mole ratios important in chemistry?

+
Mole ratios are derived from chemical equations and are essential for determining how much of each reactant is needed or produced in a reaction, which helps in stoichiometry calculations and ensuring reactions proceed as expected.
How can I determine the empirical formula from percentage composition?

+
First, convert percentages to grams assuming a 100g sample, then convert these grams to moles using molar masses. Then, divide by the smallest number of moles to find the simplest whole number ratio.