5 Simple Ways to Solve Mole Ratio Calculations

In the world of chemistry, understanding mole ratio calculations is pivotal for students, educators, and chemists alike. These calculations help in deciphering the quantitative relationship between substances in a chemical reaction. Here are five straightforward steps to master mole ratio calculations, ensuring that you can navigate through reactions with confidence and precision.
1. Understanding the Balanced Chemical Equation

Before you dive into mole ratio calculations, it’s essential to start with a balanced chemical equation. The balanced equation gives you the stoichiometric coefficients, which are crucial for determining the ratio of reactants to products. Here’s what you should do:
- Write Down the Reaction: Start with the chemical reaction you want to analyze.
- Balance the Equation: Ensure that each side of the equation has an equal number of atoms for every element.
⚠️ Note: Remember, an unbalanced equation will lead to incorrect mole ratio calculations.
2. Identify Molar Masses

Once you have your balanced equation, you’ll need the molar mass of each reactant and product. Here’s how to proceed:
- Find Atomic Masses: Use the periodic table to determine the atomic mass of each element involved.
- Calculate Molar Mass: Sum the atomic masses of each element in the compound to find the compound’s molar mass.
3. Establish Mole Ratios
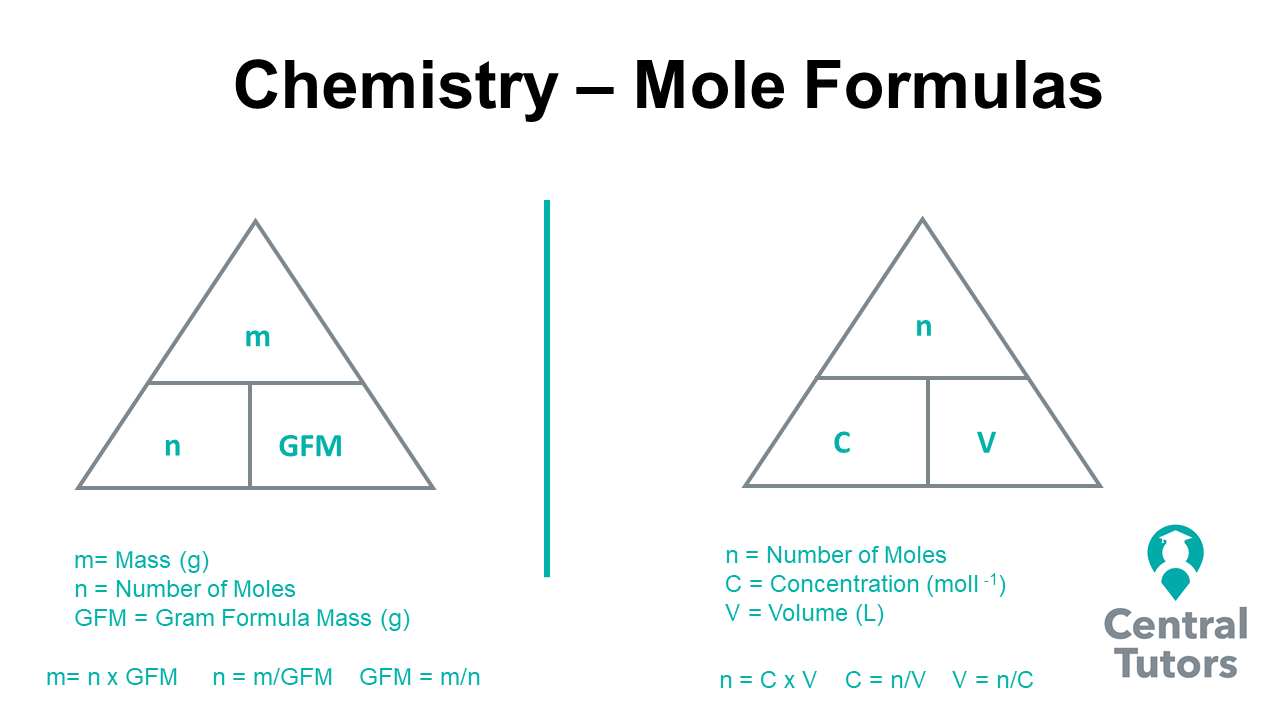
With the balanced equation and molar masses in hand, you can now establish the mole ratio. Here’s the process:
- Look at Coefficients: The coefficients from the balanced equation are your mole ratios. For example, if you have 2 moles of hydrogen gas reacting with 1 mole of oxygen gas to form water, the ratio is 2:1.
| Reactant/Product | Number of Moles |
|---|---|
| Hydrogen | 2 |
| Oxygen | 1 |
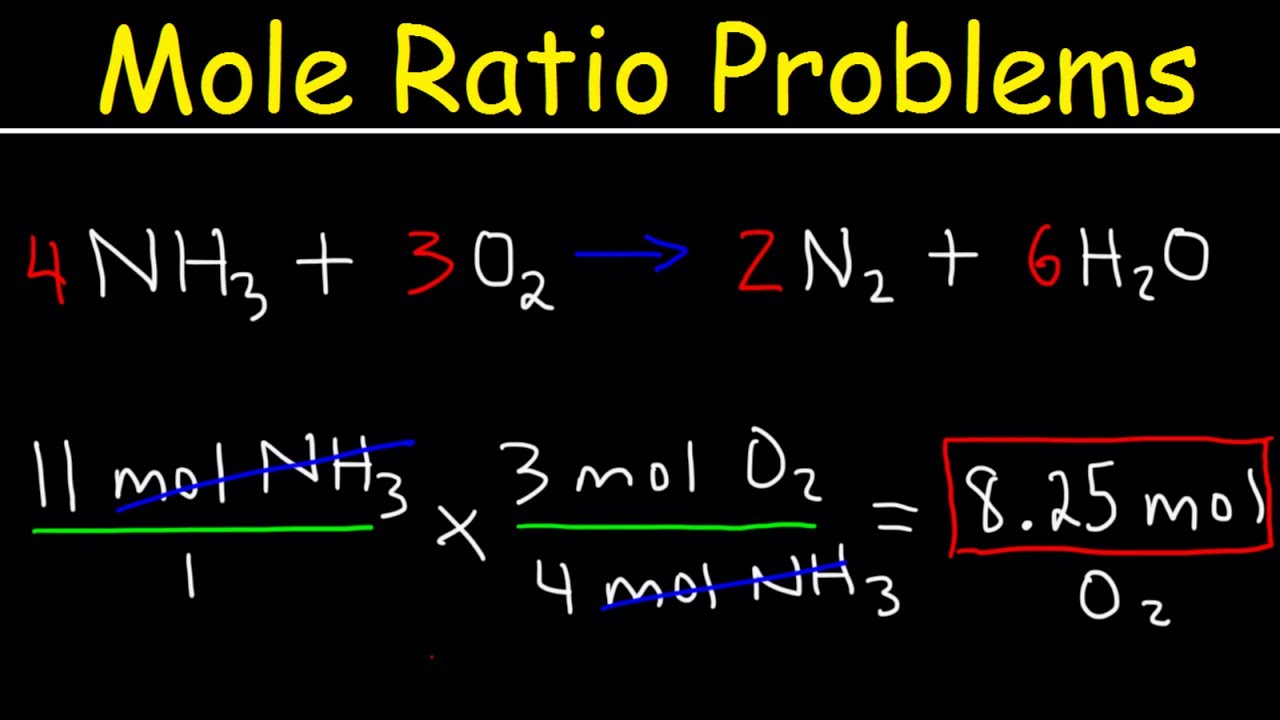
4. Using Moles to Mass Conversion

To understand how much of each substance is involved in grams, you’ll need to:
- Multiply Moles by Molar Mass: This converts moles of a substance into mass (grams).
- Divide Mass by Molar Mass: To convert mass back to moles if needed.
🔄 Note: Always check your units to ensure consistency in calculations.
5. Solving for Unknowns

When you know the amount of one substance in a reaction, you can use mole ratios to find out how much of another substance is involved. Here’s what to do:
- Set Up Proportion: Use the mole ratio from the balanced equation to set up a proportion with the known amounts.
- Solve for Unknown: Cross-multiply and solve for the unknown substance’s moles or grams.
In conclusion, mastering mole ratio calculations requires a solid foundation in understanding chemical reactions and using fundamental chemistry principles. By following these five steps, you will not only be able to calculate the amounts of substances involved in a reaction but also gain insights into the stoichiometry of chemical processes. Chemistry, like any language, has its grammar, and stoichiometry is the sentence structure. With practice and a clear understanding of these steps, you can write the perfect 'sentence' in the language of chemistry, making every reaction understandable and predictable.
Why are mole ratios important in chemistry?
+
Mole ratios are essential for understanding the quantitative relationships between reactants and products in chemical reactions. They provide the basis for predicting how much product can be formed from given reactants and for analyzing reaction efficiency.
Can I use volume instead of moles for mole ratio calculations?

+
Yes, under standard temperature and pressure (STP) conditions, one mole of any gas occupies 22.4 liters. Therefore, you can use volumes to calculate mole ratios if all gases are at STP.
How do I deal with fractions or decimals in the mole ratios?

+
Fractions or decimals in mole ratios are typically simplified to whole numbers to make calculations easier. This simplification still respects the law of conservation of mass but makes the numbers easier to work with.