Mole Particle Conversions Made Simple: Worksheet Guide

In the world of chemistry, understanding mole particle conversions is crucial for students who aim to excel in their coursework or prepare for exams like the SAT II Chemistry. Mastering these conversions not only simplifies problem-solving but also opens up the conceptual understanding of how different substances interact on a molecular level. Here's a comprehensive guide to tackle mole particle conversions using worksheet exercises.
What are Mole Particle Conversions?

Mole particle conversions are techniques used to translate the mass or volume of a substance into the number of moles, which in turn can be converted to the number of atoms, molecules, or ions. This conversion relies on Avogadro's number (6.022 x 10^23), which is the number of particles in one mole of any substance. Understanding this is fundamental for:
- Balancing chemical equations
- Calculating the yield in a chemical reaction
- Determining the concentration of solutions
Step-by-Step Guide to Mole Particle Conversions

Understanding Avogadro's Number

Start by embedding Avogadro's number into your memory. It's 6.022 x 10^23 particles per mole, and this figure is constant for any substance.
Conversion Factors

The following conversion factors are key:
- 1 mole of any substance contains Avogadro's number of particles.
- 1 mole of an element corresponds to its atomic mass in grams (e.g., 1 mole of carbon-12 is 12 grams).
- The molar mass of a compound is derived from its molecular formula by summing the atomic masses of all atoms present.
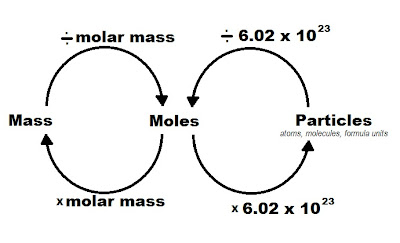
Steps to Convert Mass to Moles and Moles to Particles
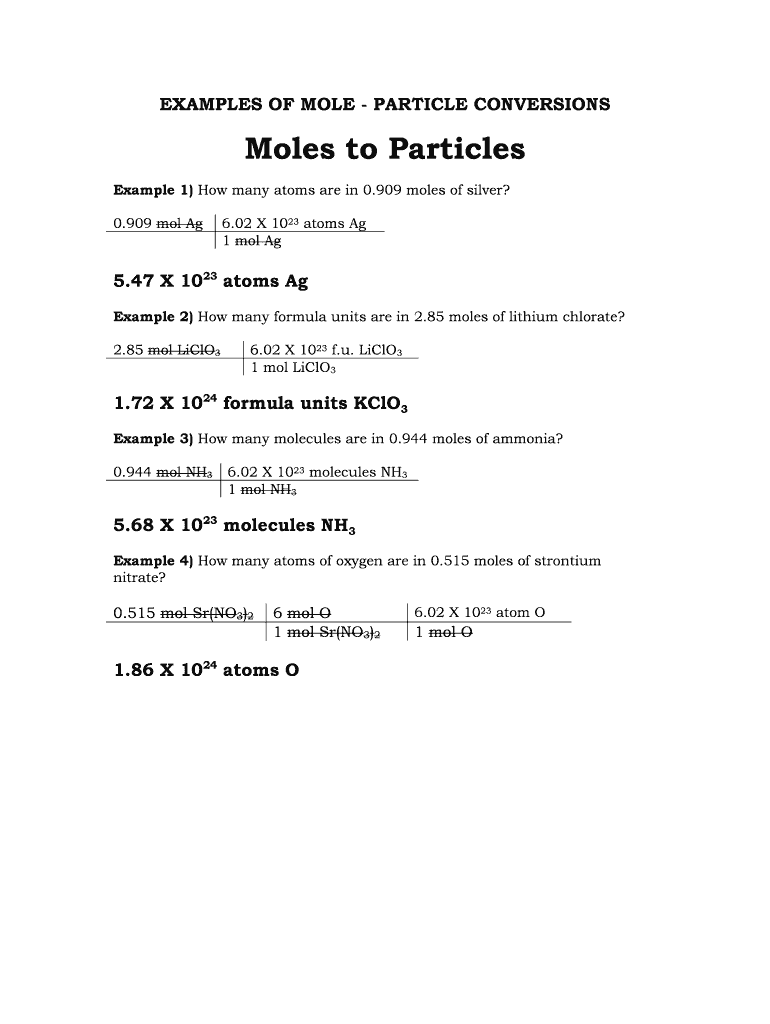
- Calculate the mass or volume: First, ensure you have the substance's mass or volume accurately measured.
- Find molar mass: Use the periodic table to determine the atomic or molecular mass.
- Convert mass to moles: Use the formula:
moles = (mass in grams) / (molar mass in grams/mole)
- Convert moles to particles: Multiply the number of moles by Avogadro's number.
particles = moles x Avogadro's number
⚠️ Note: Remember that molecular formulas change the particle conversion. For example, H2O has three times Avogadro's number of particles since each molecule contains three atoms (2H + 1O).
Worksheet Examples
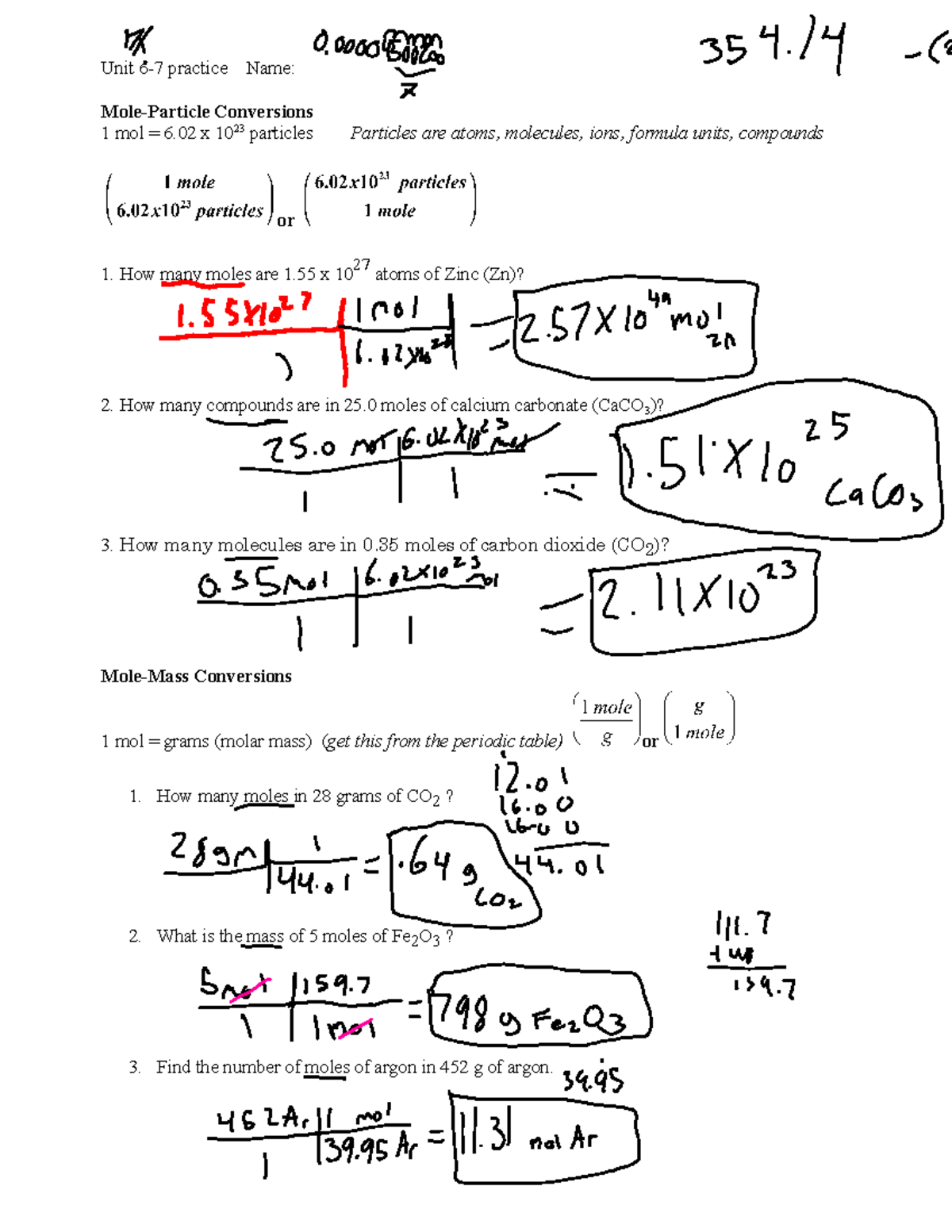
To cement your understanding, let's work through some examples you might find in a worksheet:
Example 1: Converting Mass of Water to Number of Molecules

- Mass of water provided: 18g
- Molar mass of water (H2O): 18g/mol
| Step | Calculation |
|---|---|
| Moles of Water | 18g / 18g/mol = 1 mol |
| Number of Molecules | 1 mol * 6.022 x 10^23 = 6.022 x 10^23 molecules |

Example 2: Converting Moles of Sodium to Number of Atoms

- Moles of Sodium: 2.5 mol
| Step | Calculation |
|---|---|
| Number of Atoms | 2.5 mol * 6.022 x 10^23 = 1.5055 x 10^24 atoms |
Example 3: Moles from Gas Volume
- Volume of CO2 at STP: 22.4 L
| Step | Calculation |
|---|---|
| Moles of CO2 | 22.4 L / 22.4 L/mol = 1 mol |
| Number of CO2 Molecules | 1 mol * 6.022 x 10^23 = 6.022 x 10^23 molecules |
🔔 Note: Under STP conditions (0°C and 1 atm), one mole of any ideal gas occupies 22.4 liters. However, real gases might deviate from this ideal value due to different conditions.
Conclusion

In summary, mole particle conversions are an integral part of chemistry that bridge the gap between macroscopic and microscopic understanding. By using Avogadro's number and understanding molar masses, you can accurately convert mass to moles and then to particles, whether they are atoms, molecules, or ions. Worksheets provide an excellent way to practice these conversions, ensuring you not only understand but also can apply the concepts effectively. Remember, the key to mastering these conversions lies in practice and a solid grasp of fundamental principles.
Why is Avogadro’s number significant in mole conversions?

+
Avogadro’s number (6.022 x 10^23) is the link between the macroscopic world (where we measure mass and volume) and the microscopic world (where we count atoms and molecules). It allows us to convert from moles, a more manageable unit, to the actual number of particles, which is incredibly large and impractical for direct measurement.
Can you perform mole conversions without Avogadro’s number?
+
While you can’t convert directly from moles to particles without Avogadro’s number, you can perform conversions within the mole system (like mass to moles or volume to moles) using molar mass or molar volume. Avogadro’s number is essential for particle counts.
How do temperature and pressure affect gas conversions?

+
The Ideal Gas Law shows that the volume of a gas changes with temperature and pressure. At STP (0°C, 1 atm), one mole of any ideal gas occupies 22.4 liters. However, variations in these conditions can alter this volume, necessitating adjustments in your calculations for gas conversions.